HESA Committee Report
If you have any questions or comments regarding the accessibility of this publication, please contact us at accessible@parl.gc.ca.
The Patented Medicine Prices Review Board: Ensuring the Effectiveness of the Reform Process
Introduction
The Patented Medicine Prices Review Board (the PMPRB or Board) is an independent, quasi‑judicial body, responsible for ensuring that the prices of patented medicines in Canada are not excessive during the period of market exclusivity granted to patentees. Since 2016, the Board has been working to reform its approach to evaluating drug prices. The resignations of the Acting Chairperson, a Board member and the Executive Director of the PMPRB between December 2022 and February 2023 raised questions regarding the Board’s operations and its ongoing efforts to implement drug pricing reforms. These resignations followed the release of proposed Guidelines drafted to implement regulatory changes that came into force in July 2022. The active application of those regulatory amendments is contingent on the issuance of the finalized Guidelines. Two of the people involved made public their varied motivations for resigning. Some of these reasons seemed to call into question the integrity of the Guidelines reform process.
To better understand the events that led to the three resignations, the House of Commons Standing Committee on Health (the Committee) adopted the following motion on 9 March 2023:
That pursuant to Standing Order 108(2), the committee conduct a study of the Patented Medicine Prices Review Board (PMPRB), and that the committee invite the following witnesses in addition to any further witnesses the committee may consider relevant:
- Hon. Jean-Yves Duclos, Minister of Health;
- Matthew Herder, former member, PMPRB;
- Mélanie Bourassa Forcier, former acting chair, PMPRB; and
- Douglas Clark, former executive director, PMPRB;
that the committee report its findings and recommendations to the House; and that, pursuant to Standing Order 109, the committee request that the government table a comprehensive response to the report.[1]
The Committee held two meetings on this issue (27 April 2023 and 2 May 2023), during which it heard from seven witnesses: the four witnesses named in the motion and three government officials representing the Department of Health and the Department of Justice. The Committee also received seven written briefs. Additionally, the four witnesses named in the motion dated 9 March 2023 each submitted correspondence as requested by the Committee in a 4 May 2023 motion.[2]
This report provides background information on the PMPRB’s reforms and summarizes the evidence received during the study. The report also offers the federal government recommendations to improve the effectiveness of the PMPRB’s operations and, in particular, help ensure the successful implementation of regulatory reforms.
PMPRB Reforms
The PMPRB is a quasi-judicial body that operates at arm’s length from the federal health minister. It was established in 1987 under the Patent Act.[3] It has a dual mandate: to ensure that the prices of patented medicines are not excessive during the period of market exclusivity granted to the patentees; and to provide information on drug pricing trends in the pharmaceutical industry.[4] The PMPRB reviews the factory gate price of patented medicines. Section 85 of the Patent Act sets out the factors that the Board must consider in determining whether the price of a patented medicine is excessive, such as the prices of the medicine in other countries and the prices of other medicines in the same therapeutic class.[5]
If the price of a medicine appears excessive, the PMPRB begins an investigation that may lead to a Voluntary Compliance Undertaking with the patentee or to a public hearing. If, following a hearing, the Board determines that the price is excessive, it may order the patentee to reduce the price of the medicine and offset revenues received as a result of the excessive price.[6]
A 2015 report by Health Canada’s Advisory Panel on Healthcare Innovation raised concerns over the PMPRB’s ability to curb excessive drug prices. The report recommended that the federal government “review and strengthen the PMPRB, paying particular attention to the choice of reference countries, and how PMPRB arrives at a benchmark price.”[7]
The PMPRB’s own Strategic Plan 2015–2018 noted that “[t]he coupling of relatively high patented drug prices and record low R&D [research and development] calls into question the effectiveness of the current regime in meeting its original policy objectives.”[8]
Subsequently, the PMPRB began a process to reform its Guidelines, publishing, in 2016, a paper entitled PMPRB Guidelines Modernization: Discussion Paper.[9] The Guidelines, which are authorized by section 96(4) of the Patent Act, are intended to apprise patentees of the PMPRB’s process in assessing whether a patented medicine appears to be excessively priced.[10] In 2017, the Government of Canada proposed amendments to the PMPRB’s regulatory framework.[11]
Amendments to the Patented Medicines Regulations
The Patented Medicines Regulations[12] (the Regulations) are issued under the authority of the Patent Act. Notice of proposed amendments to the Regulations was published in the Canada Gazette on 2 December 2017.[13] On 21 August 2019, following a stakeholder feedback period, the federal government published amendments to the Regulations.[14]
Among the main changes brought about through the amendments was an updated list of comparator countries. This list, referred to as the “PMPRB11,” comprises Australia, Belgium, France, Germany, Italy, Japan, the Netherlands, Norway, Spain, Sweden and the United Kingdom. In a notable contrast to the previous list of comparator countries (“PMPRB7”), the revised list removes the United States and Switzerland, the countries with the highest drug prices globally.
The amended Regulations were originally set to come into force on 1 July 2020 but were delayed four times, by six months on each occasion. According to the Government of Canada, the COVID-19 pandemic was among the reasons for the delays.[15] In August 2019, Merck Canada Inc. and six other pharmaceutical companies filed an application for judicial review with the Superior Court of Québec, challenging the validity of the special regime for protected or patented medicines set out in the Patent Act, the Regulations and the 2019 amendments.[16] In February 2022, the Court of Appeal of Quebec issued a decision upholding the constitutionality of the existing regime and revised list of comparator countries, while holding other regulatory amendments to be invalid.[17] The Attorney General of Canada did not seek leave to appeal the decision to the Supreme Court of Canada.[18]
In April 2022, the Minister of Health (the Minister) announced that the federal government would implement the updated list of comparator countries and reduced reporting requirements for those medicines at lowest risk of excessive pricing but would repeal the amendments held invalid in the Merck decision.[19] The amended Regulations came into force on 1 July 2022.[20] However, the PMPRB will not apply those amendments until it has issued its finalized Guidelines.[21]
PMPRB 2022 Draft Guidelines
Under section 96 of the Patent Act, the PMPRB is authorized to issue non-binding guidelines once it has consulted with the federal health minister, the provincial ministers of health, and “such representatives of consumer groups and representatives of the pharmaceutical industry as the [federal health minister] may designate for the purpose.”[22]
On 6 October 2022, the PMPRB published a draft of its new Guidelines, which were intended to give effect to the amended Regulations that had come into force on 1 July 2022.[23] It also opened a 60-day consultation period.[24] At the time, the PMPRB had anticipated that these Guidelines would come into effect on 1 January 2023. It held webinars on the topic and received 88 written submissions during the consultation period, which closed on 5 December 2022. At the time of the drafting of this report, the new Guidelines had not yet been implemented.
Letters to the Acting Chairperson
On 18 November 2022, the president of Innovative Medicines Canada (IMC), an industry stakeholder group, sent a letter to the Acting Chairperson of the PMPRB expressing concerns about the proposed Guidelines and requesting a meeting.[25]
On 28 November 2022, the Minister sent a letter to the Acting Chairperson. In this letter, the Minister noted the following:
[T]his new version of the Guidelines signal a pivotal change from a long-standing practice of including price tests and price ceilings, to instead including investigation criteria.
Given the new direction set out in the proposed new Guidelines, it is critical that all stakeholders understand fully how the new Guidelines will be implemented. Many stakeholders have raised concerns and questions associated with the new Guidelines, and are looking for more information about the potential impacts and on the operationalization of some of the key technical aspects of the Guidelines. It is only with this more detailed understanding that stakeholders can engage meaningfully in the consultation process. In parallel, the Board will benefit in receiving the considered views and feedback of stakeholders as part of its decision‑making.[26]
The Minister requested that the Board “consider pausing [‘envisager de suspendre’] the consultation process, so as to allow time to work collaboratively, with all stakeholders, to understand fully the short and long‑term impacts of the proposed new Guidelines.”[27]
Resignations From the PMPRB
On 5 December 2022, Mélanie Bourassa Forcier resigned as acting chairperson of the PMPRB. At the time, she declined to comment on her resignation, citing legal constraints,[28] but on 3 March 2023 she published a letter explaining her reasons for resigning.[29] In it, she related the concern that she felt in light of the letters from the Minister and IMC and what she viewed as insufficient stakeholder consultation regarding the proposed Guidelines. She resigned on the last day of the scheduled consultation period, having disagreed with the position taken by the other Board members and the Executive Director with respect to the handling of the consultation process. In the letter, she expressed the opinion that the PMPRB was not fulfilling its obligations and that the decision not to extend the consultation period created the risk of litigation and potentially jeopardized the accessibility of medications in Canada.
On 20 February 2023, Matthew Herder resigned from his position as Board member, explaining his decision in a public letter.[30] In this letter, he claimed that the government had repeatedly failed to follow through on implementing PMPRB reforms. He outlined three main reasons for his resignation: from his perspective, the government had failed to defend policy changes in court, delayed the coming into force of the new Regulations, and undermined the PMPRB’s independence and credibility. In his view, the Minister had intervened in the PMPRB’s independent process by requesting that the Guidelines consultation process be suspended.
On 24 February 2023, the PMPRB announced that Douglas Clark would be stepping down as executive director.[31] At that time, no reason was shared publicly for his resignation.
Figure 1 shows the PMPRB organizational chart as it appeared prior to the resignations. The Minister appointed a new chairperson, Thomas J. Digby, on 1 February 2023.[32]
Figure 2 presents a timeline of key events in the PMPRB reform process.
Figure 1—PMPRB Organizational Chart, 2021
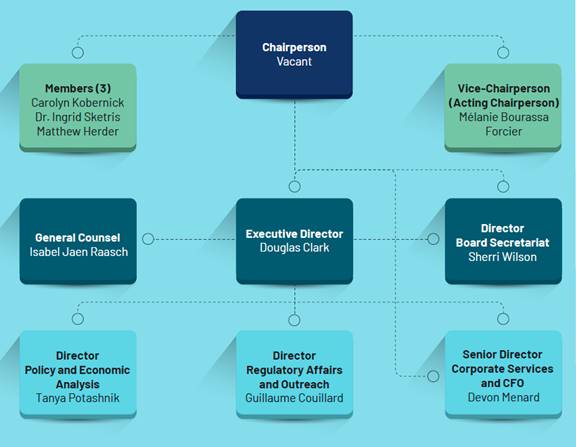
Source: PMPRB, Annual Report 2021.
Figure 2—Timeline of Key Events in PMPRB Reforms

Source: Figure created by the Library of Parliament.
Discussion of Events That Led up to the Resignations
The evidence gathered during this study centred on events that occurred during the fall 2022 consultation period for the PMPRB’s draft Guidelines and the subsequent resignations of the Acting Chairperson, a Board member and the Executive Director. Among the themes that emerged from the testimony were allegations of ministerial interference in the PMPRB’s Guidelines consultation process; the pharmaceutical industry’s influence on Canadian drug policy; and issues with the PMPRB’s operating procedures.
Allegations of Ministerial Interference
In his letter of resignation addressed to Minister of Health Jean‑Yves Duclos, Matthew Herder claimed that the Minister’s request to suspend the Guidelines consultation process “undermined the Board’s credibility and interfered with the exercise of a function that goes to the very heart of its expertise as an independent, arms-length administrative tribunal.”[33] More broadly, Matthew Herder told the Committee that the interactions between the PMPRB and Health Canada during the 2022 Guidelines consultation process represented a “dramatic change” from previous communication patterns during his tenure.[34] The absence of meetings between the Minister and the Board, as well as the intent and impact of the Minister’s letter to the Acting Chairperson, were both central points of discussion in the testimony.
Absence of Meetings Between the PMPRB and the Minister of Health
The Committee heard that PMPRB and Health Canada officials routinely hold working‑level briefings.[35]
Regarding interactions between the Minister and the PMPRB, witnesses referenced two provisions of the Patent Act. Section 102 concerns meetings between the Minister and the Board and reads as follows:
- (1) The Minister may at any time convene a meeting of the following persons:
- a) the Chairperson and such members of the Board as the Chairperson may designate;
- b) the provincial ministers of the Crown responsible for health or such representatives as they may designate;
- c) such representatives of consumer groups and representatives of the pharmaceutical industry as the Minister may designate; and
- d) such other persons as the Minister considers appropriate.[36]
Under section 96 of the Patent Act, the Minister is one of the parties the PMPRB must consult with before it issues guidelines:
- (4) Subject to subsection (5), the Board may issue guidelines with respect to any matter within its jurisdiction but such guidelines are not binding on the Board or any rights holder or former rights holder.
- Consultation
- (5) Before the Board issues any guidelines, it shall consult with the Minister, the provincial ministers of the Crown responsible for health and such representatives of consumer groups and representatives of the pharmaceutical industry as the Minister may designate for the purpose.[37]
There was a lack of clarity among witnesses as to which party should or could initiate contact. The Minister stated, “[I]t would have been inappropriate for me to want to contact the chairperson, unless [the chairperson] had set things in motion.”[38] For her part, Mélanie Bourassa Forcier said, “I was told that to meet the minister, I would have to wait for him to invite me. I therefore never met him because I never received an invitation.”[39] She indicated that she had never once met with the Minister during her tenure as acting chairperson, between November 2021 and December 2022, despite having made multiple requests to PMPRB staff to arrange such a meeting, including when she first took office.[40] The Minister did not address the import of section 102(1) of the Patent Act.
Douglas Clark, who served as executive director of the PMPRB for 10 years, stated that, during his tenure, the deputy minister’s office typically initiated and arranged briefings, “often at the behest of the minister’s office.”[41] He explained that, as executive director, he would sometimes be called upon to take on certain duties on behalf of the chairperson. As Douglas Clark told the Committee, “[W]ith the exception of the current minister, I have personally briefed every minister of Health on guidelines reform as far back as Minister Ambrose under the previous government, either on behalf of the chairperson or together.”[42]
Mélanie Bourassa Forcier stated that only the chairperson is to be in contact with the federal health minister, according to the Chair’s Guidelines for the Conduct of Board Members (Chair’s Guidelines), and that the PMPRB organizational chart makes no link between the executive director and that minister.[43] Article 35 of the Chair’s Guidelines stipulates that “Board Members, other than the Chairperson, should strive to minimize contact with Members of Parliament, Ministers, political staff and public servants outside of Board Staff, on any matters relating to the PMPRB.”[44]
Douglas Clark told the Committee about his unsuccessful attempts to arrange a meeting with the Minister. He described making “multiple overtures to the minister’s chief of staff and senior policy adviser via texts, emails and phone calls,” followed by a lack of response from department officials.[45] Documentation of these communications, occurring between 9 November 2022 and 21 November 2022, was included in Douglas Clark’s submission to the Committee.[46]
Mélanie Bourassa Forcier also told the Committee about unsuccessful attempts to arrange a meeting with the Minister, attempts that she contended had been met with resistance by PMPRB staff for reasons to do with “following the proper reporting structure.”[47] She described her multiple requests to arrange a meeting with the Minister after she realized that there was a “communications problem between Health Canada and the PMPRB.”[48] According to her submission to the Committee, she had not been made aware of Douglas Clark’s attempts to reach out to the Minister.[49] Douglas Clark denied that he had kept information from the Acting Chairperson or that PMPRB staff had resisted requests to arrange a meeting between her and the Minister.[50]
Correspondence submitted to the Committee by Mélanie Bourassa Forcier shows that, on 18 November 2022, she had asked Douglas Clark to arrange a meeting with the Minister.[51] She made a similar request to the Board’s executive secretary on 21 November 2022.[52] Mélanie Bourassa Forcier also requested a meeting with the Minister in her 30 November 2022 response to the Minister’s letter.[53]
On 30 November 2022, Mélanie Bourassa Forcier and Douglas Clark met with Deputy Minister Steven Lucas.
Reactions to the Minister of Health’s Letter
The Minister’s letter requesting that the Board consider pausing (or suspending) the Guidelines consultation process came as a surprise to the witnesses who had been working for the PMPRB at the time.[54] Their views differed, however, as to the appropriateness and intent of the letter.
The Honourable Jean-Yves Duclos stated that the letter was sent in the context of consultations on the draft Guidelines, pursuant to section 96(5) of the Patent Act, which sets out the PMPRB’s duty to consult with the federal health minister, among other parties, before the Board issues guidelines.[55]
Douglas Clark told the Committee that the content of the letter had been of “grave concern” to himself, as well as to the PMPRB’s senior staff and general counsel.[56] He said that the request had surprised him, since Health Canada staff had seemed supportive of the policy approach. He testified that PMPRB personnel had briefed Health Canada staff seven times between 4 October 2022 and 25 November 2022 and that Health Canada had expressed no concerns about the draft Guidelines then.[57] Additionally, Douglas Clark told the Committee that he, along with other senior PMPRB staff, had met with IMC and approximately 20 industry representatives to discuss the draft Guidelines on 23 November 2022.[58]
Mélanie Bourassa Forcier responded to the Minister’s request in a letter dated 30 November 2022. In her letter, she expressed her surprise at the concerns that were raised, having previously thought that Health Canada was “comfortable and in agreement with the approach.” She also highlighted the consultation efforts that had already been made:
[T]he PMPRB has from the start communicated the existence of these consultations to [Health Canada] and all provincial health departments and held follow-up meetings with provincial health department officials and with the Pan-Canadian Pharmaceutical Alliance (pCPA). We have also hosted webinars for the pharmaceutical industry and held lengthy meetings with Innovative Medicines Canada (IMC) and multiple IMC member corporations.[59]
In his resignation letter, Matthew Herder contended that the Minister’s request had interfered with the PMPRB’s independence and undermined its credibility.[60] He told the Committee that he had interpreted the request as being “a very strong suggestion, if not a demand.”[61] In his view, some of the language in the letter echoed industry talking points, and he noted that the PMPRB had received a similar request from a pharmaceutical industry group. The letter, he said, was “incredibly divisive inside the Board.”[62]
There was fundamental disagreement among Board members as to how the PMPRB should respond to the Minister’s request. Mélanie Bourassa Forcier posited that some within the PMPRB may have interpreted the concept of “suspending” too narrowly to mean “halting” or “stopping” as opposed to “extending” the consultation process.[63] She explained that she had wanted to better understand the potential impacts of the Guidelines on stakeholders and address any possible legal issues.[64] Further, Mélanie Bourassa Forcier stressed the importance of ensuring adequate time for consultation with stakeholders in accordance with section 96(5) of the Patent Act, particularly given the concerns expressed by industry and patient groups about the draft Guidelines. She had proposed two options to Board members: a meeting with IMC by the end of the consultation period, or the suspension or extension of the consultation period pending such a meeting.[65]
The other Board members had wanted the consultation process to run its course, ending as planned on 5 December 2022, following which the Board would decide at its quarterly meeting on 13 December 2022 how it wished to proceed with the Guidelines and whether an additional consultation period was warranted.[66] In the view of the other Board members, further communication with the Deputy Minister about plans for the Guidelines consultation process could wait until after the Board’s quarterly meeting. They also expressed openness to the option of meeting with IMC on a recurring basis, as the IMC president had proposed in her letter to the Acting Chairperson of the PMPRB on 18 November 2022.[67]
In Mélanie Bourassa Forcier’s estimation, there was no interference from the Minister.[68] She affirmed that her resignation on 5 December 2022 was not directly related to the Minister’s letter. In discussing her reasons for resigning, she highlighted her discomfort with the Board’s decision “to stay silent in the face of the minister’s request, and to propose a meeting with IMC in 2023.”[69] Further, a meeting with stakeholders after the end of the consultation period would have been, in her eyes, “in direct contravention with the principles of fundamental justice and procedural fairness.”[70]
The Pharmaceutical Industry’s Influence on Drug Policy
The pharmaceutical industry’s opposition to the 2022 draft Guidelines, as well as to previous consultations on draft Guidelines, was another theme in the testimony. Several witnesses noted the industry’s resistance to engaging with the proposed changes.[71] In a briefing note to the Minister on 8 December 2021, Mélanie Bourassa Forcier wrote that, “[a]fter 5 years, myriad policy proposals and many hundreds of hours of consultation, it would appear that the pharmaceutical industry is simply not amenable to any measure that would further constrain its ability to sell patented medicines in Canada at free market prices.”[72]
In his testimony, Matthew Herder emphasized the industry’s power to influence pharmaceutical policy in Canada. The Committee heard that the pharmaceutical industry has far more resources at its disposal than the PMPRB, making it hard for the latter to implement reforms that would have the effect of reducing the industry’s revenue. As Douglas Clark put it: “The PMPRB is the David to the Goliath of a transnational trillion‑dollar industry.”[73]
According to Matthew Herder, the industry’s influence seems to extend to Health Canada. He suggested that this influence had prompted the Minister’s request to suspend the PMPRB consultation: “Industry now knows it can bypass the PMPRB when it isn’t satisfied with the board’s policy direction and can get the minister to do its bidding.”[74] Further, he stated that there “appear to be direct lines of communication between Health Canada and industry.”[75] Some witnesses discussed the number of meetings that had occurred between pharmaceutical lobbyists and the department during the Guidelines consultation period. Correspondence submitted by Douglas Clark and Matthew Herder included records from the lobbyist registry showing that the department had met with pharmaceutical interest groups and companies at least 13 times between October and December 2022.[76] When asked about the number of times he had met with lobbyists, the Honourable Jean-Yves Duclos told the Committee, “I have met those members of the industry, including exporters, manufacturers and developers, for all sorts of reasons, including addressing the analgesics shortages that we saw.”[77]
Some witnesses suggested that the PMPRB Guidelines reform could threaten investments in drug manufacturing capacity or the supply of drugs during a pandemic. Douglas Clark opined that such a fear may have meant that “the imperative of smoothing out relations with the industry trumped any consideration of whether the guidelines were sound policy or had merit.”[78] When asked whether she was concerned that pharmaceutical companies might limit access to pandemic vaccines if the PMPRB reforms were to go ahead, Mélanie Bourassa Forcier said, “Yes I was worried about that.”[79]
The Committee heard testimony regarding the industry’s influence on public opinion regarding the PMPRB reforms, with certain witnesses expressing concerns about inaccuracies or misrepresentation in pharmaceutical industry messaging. According to Matthew Herder, more should be done to counteract such misleading information: “Industry plays fast and loose with the facts because they can and because we let them.”[80]
Witnesses also discussed potential conflicts of interest between Board members and the pharmaceutical industry. From Mélanie Bourassa Forcier’s point of view, having a PMPRB chairperson with industry expertise, such as the current chairperson, is an asset rather than an issue.[81] For his part, Matthew Herder considered it problematic when Board members assume industry roles at the end of their terms.[82] He argued that conflicts of interest between the PMPRB and industry need to be addressed: “Unless we start taking conflicts of interest far more seriously, meaningful pricing reform will be impossible.”[83]
Issues With the PMPRB’s Operating Procedures
Mélanie Bourassa Forcier’s testimony pointed to potential operational problems at the PMPRB, which may have precipitated the deterioration of communications among Board members and with Health Canada and other stakeholders. According to her submission, a “lack of internal operating rules,” particularly as to how the role of executive director was understood, created challenges.[84] As she saw it, internal practices had evolved over time to the point of varying from those set out in the Patent Act, the Chair’s Guidelines and the PMPRB organizational chart. Mélanie Bourassa Forcier recommended that the PMPRB’s procedures be reviewed or clarified in several areas.[85]
The correspondence submitted reveals a lack of clarity among Board members over certain internal procedures. For example, when her position diverged from that of the Executive Director and of the other members regarding how to respond to the Minister’s letter, Mélanie Bourassa Forcier determined that she alone would make the decision to suspend the consultation process, on the basis of her interpretation of her powers under the Patent Act.[86] Legal counsel was sought on the question of whether the chairperson could “independently and unilaterally decide substantive matters related to the issuance of and consultation on Board Guidelines.”[87] According to the resulting legal opinion, the chairperson’s powers are limited to “internal day‑to‑day administrative management matters, and … do not supersede the powers and authorities granted to the Board as a whole.”[88] Legal advice was also sought on several other issues related to the applicable rules and obligations of the chairperson and other Board members, such as “obligations of confidentiality relating to Board discussions on the proposed guidelines.”[89]
Mélanie Bourassa Forcier’s testimony stressed the importance of meaningful dialogue with industry and other stakeholders. She raised concerns about impartiality within the Board. Board members, she explained to the Committee, should take a neutral approach in their duty to consult with stakeholders. She posited that the multiple delays in the implementation of the reforms had created tension between the Executive Director and the Minister’s office, and that the setbacks in the reform process had contributed to a long‑standing “dialogue of the deaf” between the PMPRB and its stakeholders.[90]
Mélanie Bourassa Forcier noted that Board members had not been made aware of the contents of stakeholders’ submissions prior to the close of the consultation period and that some of those submissions had contained requests for a time extension.[91] She therefore recommended that members “have timely access to the contents of submissions presented in consultations.”[92] In an email to Board members, Douglas Clark pointed out that the 2022 consultation had “followed the same protocol as the previous two rounds of consultations on proposed new Guidelines in 2020.”[93] In his view, any decision to re-open the consultation process or initiate a new one should be made only once the current consultation period had come to its scheduled end and the Board had been afforded the opportunity to assess the entirety of the feedback received.
Witnesses discussed the PMPRB more broadly—its function, responsibilities, operating practices and mandate. The Minister highlighted the Government of Canada’s support and respect for the Board’s role in protecting consumers from excessive drug prices.[94] He also raised the need to balance drug affordability against R&D and medicine availability in Canada.[95] Mélanie Bourassa Forcier highlighted the importance of industry’s role in R&D and in bringing innovative medicines to the market. She felt that the federal government could do more to support innovation and the accessibility of medicines. Further, she suggested that the Board’s mandate should be clarified: “[I]s the PMPRB’s role strictly to ensure that the price of patented medicines is not excessive, or is it a body that ensures access to medicines at a price that is not excessive?”[96]
Finally, the testimony stressed the importance of preserving the PMPRB’s independence. The Honourable Jean-Yves Duclos noted that, whereas the health minister is responsible for the patented medicine pricing provisions of the Patent Act and the regulatory framework governing patented medicines, the PMPRB has the authority to issue non‑binding guidelines following consultations with certain stakeholders.[97] In her letter to the Minister, Mélanie Bourassa Forcier noted that issuing non-binding guidelines “is a function that is central to the expertise and autonomy of the Board.”[98] For his part, Matthew Herder affirmed that the Board must “remain the master of [its] own guidelines … . Certainly there can be no interference. We need a recommitment to independence.”[99]
Conclusion and Recommendations
The Committee heard contrasting accounts of the events that led up to the resignations of the Acting Chairperson, a Board member and the Executive Director of the PMPRB around the time of the 2022 draft Guidelines consultation period. Witnesses were divided on the reasons behind the collapse of the consultation process. Some blamed pressures exerted on the Board by the Minister of Health and pharmaceutical industry, while others refuted the existence of external interference and pointed to problems within the PMPRB, such as resistance to meaningful stakeholder engagement or ambiguities in operating procedures.
Seven years after its initiation, the PMPRB’s reform process remains incomplete; until the new Guidelines are issued, the regulatory amendments that came into force in July 2022 will not be operationalized. To enable the PMPRB to more effectively carry out its mandate of protecting Canadian consumers against excessive drug pricing and ensure that the Board is successful in reforming its approach to evaluating drug prices, the Committee puts forward the following recommendations:
Recommendation 1
That the Government of Canada implement a clear communications protocol between the Minister of Health and the Chairperson and members of the Patented Medicine Prices Review Board.
Recommendation 2
That the Government of Canada review the process by which it enacts Patented Medicine Prices Review Board regulatory reform.
Recommendation 3
That the Government of Canada review the way in which it interacts with the pharmaceutical industry as a regulated sector with monopolistic patent pricing power.
Recommendation 4
That the mandate of the Patented Medicine Prices Review Board be clarified. The Board should be mandated to ensure that the prices of patented medicines are not excessive while also ensuring that pricing does not ultimately limit patient access, particularly in the case of rare diseases.
Recommendation 5
That the Patented Medicine Prices Review Board review its internal operating rules to ensure that they are clear and transparent. In addition, members appointed to the organization should be provided with independent, external support to help them in the event of misunderstandings and issues.
Recommendation 6
That the Patented Medicine Prices Review Board consider case studies to gain insight into how its future Guidelines would be applied in practice and to have a firmer idea of their potential consequences on patients and the life sciences ecosystem.
Recommendation 7
That the Patented Medicine Prices Review Board include a broader range of stakeholders in policy development.
Recommendation 8
That the members of the Patented Medicine Prices Review Board always have real‑time access to the contents of submissions presented in consultations.
Recommendation 9
That a registry be created to track drug penetration rates in Canada and compare them with similar countries.
Recommendation 10
That the Government of Canada maintain a public registry of publicly funded innovations, alone or in partnership with the industry, and ensure that what it funds is available in the Canadian marketplace.
[1] House of Commons, Standing Committee on Health [HESA], Minutes of Proceedings, 9 March 2023.
[2] HESA, Minutes of Proceedings, 4 May 2023.
[3] Patent Act, R.S.C. 1985, c. P-4.
[4] Patented Medicine Prices Review Board [PMPRB], Mandate and Jurisdiction.
[5] Patent Act, R.S.C. 1985, c. P-4, s. 85.
[6] Government of Canada, “Regulatory Process,” Patented Medicine Prices Review Board.
[7] Health Canada, Unleashing Innovation: Excellent Healthcare for Canada: Report of the Advisory Panel on Healthcare Innovation, July 2015.
[8] PMPRB, Strategic Plan 2015–2018.
[9] PMPRB, PMPRB Guidelines Modernization: Discussion Paper, June 2016.
[10] Government of Canada, PMPRB Guidelines.
[11] Regulations Amending the Patented Medicines Regulations, Canada Gazette, Part I, 2 December 2017.
[12] Patented Medicines Regulations, SOR/94-688.
[13] Regulations Amending the Patented Medicines Regulations, Canada Gazette, Part I, 2 December 2017.
[14] Regulations Amending the Patented Medicines Regulations (Additional Factors and Information Reporting Requirements): SOR/2019-298, 8 August 2019, Canada Gazette, Part II, 21 August 2019.
[15] Regulations Amending the Regulations Amending the Patented Medicines Regulations (Additional Factors and Information Reporting Requirements), No. 5: SOR/2022-162, Canada Gazette, Part II, 6 July 2022.
[16] Merck Canada inc. c. Procureur général du Canada, 2020 QCCS 4541 (CanLII); and Regulations Amending the Regulations Amending the Patented Medicines Regulations (Additional Factors and Information Reporting Requirements), No. 5: SOR/2022-162, Canada Gazette, Part II, 6 July 2022.
[17] Merck Canada inc. c. Procureur général du Canada, 2022 QCCA 240 (CanLII).
[18] See also Innovative Medicines Canada v. Canada (Attorney General), 2022 FCA 210, upholding the validity of the revised list of comparator countries.
[19] Health Canada, Statement from Minister of Health on the Coming-into-Force of the Regulations Amending the Patented Medicines Regulations, 14 April 2022.
[20] Regulations Amending the Regulations Amending the Patented Medicines Regulations (Additional Factors and Information Reporting Requirements), No. 5: SOR/2022-162, Canada Gazette, Part II, 6 July 2022.
[21] HESA, Evidence, 2 May 2023, 1135 (Douglas Clark, Executive Director, Patented Medicine Prices Review Board).
[22] Patent Act, R.S.C. 1985, c. P-4, ss. 96(4)–96(5).
[23] PMPRB, Backgrounder: PMPRB Draft Guidelines Consultation, 2022, p. 2.
[24] Government of Canada, “Draft Guidelines 2022,” 2022 Proposed updates to the PMPRB Guidelines.
[25] Innovative Medicines Canada, Letter to Dr. Mélanie Bourassa Forcier, Interim Chair, Patented Medicine Prices Review Board (PMPRB), 18 November 2022.
[26] Government of Canada, Letter to the acting Chairperson and Chief Executive Officer of the Patented Medicine Prices Review Board, 28 November 2022.
[27] Government of Canada, Letter to the acting Chairperson and Chief Executive Officer of the Patented Medicine Prices Review Board, 28 November 2022. The original letter was sent in French only. An unofficial English translation was shared with Board members on 29 November 2022 along with the original. An official translation was posted on Health Canada’s website on 13 March 2023. Both translations used the term “pausing.”
[29] Mélanie Bourassa Forcier, Les raisons de ma démission du Conseil d’examen du prix des médicaments brevetés, 3 March 2023.
[30] Matthew Herder, Re: Letter of Resignation, 20 February 2023.
[31] PMPRB, Douglas Clark stepping down as Executive Director of the PMPRB, 24 February 2023.
[32] Health Canada, Government of Canada announces appointment to the Patented Medicine Prices Review Board, 1 February 2023.
[33] Matthew Herder, Re: Letter of Resignation, 20 February 2023.
[34] HESA, Evidence, 2 May 2023, 1200 (Matthew Herder, Director, Health Law Institute, Dalhousie University, As an Individual).
[35] HESA, Evidence, 27 April 2023, 1145 (Eric Bélair, Associate Assistant Deputy Minister, Strategic Policy Branch, Department of Health); and HESA, Evidence, 2 May 2023, 1105 (Douglas Clark).
[36] Patent Act, R.S.C. 1985, c. P-4, s. 102.
[37] Patent Act, R.S.C. 1985, c. P-4, ss. 96(4)–96(5).
[39] HESA, Evidence, 27 April 2023, 1220 (Mélanie Bourassa Forcier, Full Professor, As an Individual).
[40] Correspondence submitted by Mélanie Bourassa Forcier pursuant to the motion adopted by the committee on Thursday, May 4, 2023, III. Clarification pertaining to testimony.
[43] Correspondence submitted by Mélanie Bourassa Forcier pursuant to the motion adopted by the committee on Thursday, May 4, 2023, III. Clarification pertaining to testimony.
[44] Correspondence submitted by Douglas Clark pursuant to the motion adopted by the committee on Thursday, May 4, 2023, Part II, Chair’s Guidelines for the Conduct of Board Members [p. 92 of the PDF].
[46] Correspondence submitted by Douglas Clark pursuant to the motion adopted by the committee on Thursday, May 4, 2023, Tab 7.
[49] Correspondence submitted by Mélanie Bourassa Forcier pursuant to the motion adopted by the committee on Thursday, May 4, 2023, III. Clarification pertaining to testimony [p. 9 of the PDF].
[51] Correspondence submitted by Mélanie Bourassa Forcier pursuant to the motion adopted by the committee on Thursday, May 4, 2023, Annex C1.
[52] Correspondence submitted by Mélanie Bourassa Forcier pursuant to the motion adopted by the committee on Thursday, May 4, 2023, Annex C2.
[53] Correspondence submitted by Mélanie Bourassa Forcier pursuant to the motion adopted by the committee on Thursday, May 4, 2023, Annex B.
[54] HESA, Evidence, 2 May 2023, 1110 (Douglas Clark), Correspondence submitted by Douglas Clark pursuant to the motion adopted by the committee on Thursday, May 4, 2023, Tab 5.
[57] HESA, Evidence, 2 May 2023, 1145 (Douglas Clark); Correspondence submitted by Douglas Clark pursuant to the motion adopted by the committee on Thursday, May 4, 2023, Tab 6.
[58] Correspondence submitted by Douglas Clark pursuant to the motion adopted by the committee on Thursday, May 4, 2023, Part II [p. 109 of the PDF]; and HESA, Evidence, 2 May 2023, 1110 (Douglas Clark).
[59] Correspondence submitted by Mélanie Bourassa Forcier pursuant to the motion adopted by the committee on Thursday, May 4, 2023, Annex B.
[60] Matthew Herder, Re: Letter of Resignation, 20 February 2023.
[63] Correspondence submitted by Mélanie Bourassa Forcier pursuant to the motion adopted by the committee on Thursday, May 4, 2023, III. Clarification pertaining to testimony.
[64] Correspondence submitted by Mélanie Bourassa Forcier pursuant to the motion adopted by the committee on Thursday, May 4, 2023, III. Clarification pertaining to testimony.
[65] Correspondence submitted by Mélanie Bourassa Forcier pursuant to the motion adopted by the committee on Thursday, May 4, 2023, III. Clarification pertaining to testimony [p. 15 of the PDF]; and Correspondence submitted by Douglas Clark pursuant to the motion adopted by the committee on Thursday, May 4, 2023, Part II [p. 130 of PDF].
[66] Correspondence submitted by Mélanie Bourassa Forcier pursuant to the motion adopted by the committee on Thursday, May 4, 2023, Annex F; and Correspondence submitted by Douglas Clark pursuant to the motion adopted by the committee on Thursday, May 4, 2023, Part II.
[67] Correspondence submitted by Matthew Herder pursuant to the motion adopted by the committee on Thursday, May 4, 2023, B1.
[68] Correspondence submitted by Mélanie Bourassa Forcier pursuant to the motion adopted by the committee on Thursday, May 4, 2023, Conclusion; and HESA, Evidence, 27 April 2023, 1225 (Mélanie Bourassa Forcier).
[69] Correspondence submitted by Mélanie Bourassa Forcier pursuant to the motion adopted by the committee on Thursday, May 4, 2023, III. Clarification pertaining to testimony.
[70] Correspondence submitted by Mélanie Bourassa Forcier pursuant to the motion adopted by the committee on Thursday, May 4, 2023, III. Clarification pertaining to testimony.
[71] HESA, Evidence, 2 May 2023, 1205 (Douglas Clark); HESA, Evidence, 2 May 2023, 1140 (Matthew Herder); and HESA, Evidence, 27 April 2023, 1235 (Mélanie Bourassa Forcier).
[72] Correspondence submitted by Douglas Clark pursuant to the motion adopted by the committee on Thursday, May 4, 2023, Tab 9.
[76] Correspondence submitted by Douglas Clark pursuant to the motion adopted by the committee on Thursday, May 4, 2023, Tab 10; and Correspondence submitted by Matthew Herder pursuant to the motion adopted by the committee on Thursday, May 4, 2023, D1.
[81] Mélanie Bourassa Forcier, Les raisons de ma démission du Conseil d’examen du prix des médicaments brevetés, 3 March 2023.
[84] Correspondence submitted by Mélanie Bourassa Forcier pursuant to the motion adopted by the committee on Thursday, May 4, 2023, III. Clarification pertaining to testimony.
[86] Correspondence submitted by Matthew Herder pursuant to the motion adopted by the committee on Thursday, May 4, 2023, B3.
[87] Correspondence submitted by Douglas Clark pursuant to the motion adopted by the committee on Thursday, May 4, 2023, Part II [p. 81 of the PDF].
[88] Correspondence submitted by Douglas Clark pursuant to the motion adopted by the committee on Thursday, May 4, 2023, Part II [p. 81 of the PDF].
[89] Correspondence submitted by Douglas Clark pursuant to the motion adopted by the committee on Thursday, May 4, 2023, Part II [p. 99 of the PDF].
[90] Correspondence submitted by Mélanie Bourassa Forcier pursuant to the motion adopted by the committee on Thursday, May 4, 2023, III. Clarification pertaining to testimony; and Mélanie Bourassa Forcier, Les raisons de ma démission du Conseil d’examen du prix des médicaments brevetés, 3 March 2023.
[91] Correspondence submitted by Mélanie Bourassa Forcier pursuant to the motion adopted by the committee on Thursday, May 4, 2023, III. Clarification pertaining to testimony.
[93] Correspondence submitted by Douglas Clark pursuant to the motion adopted by the committee on Thursday, May 4, 2023, Part II [p. 122 of the PDF].