ENVI Committee Report
If you have any questions or comments regarding the accessibility of this publication, please contact us at accessible@parl.gc.ca.
8. RISK AND VALUE ASSESSMENTS
8.1 One of the primary missions of the Pest Management Regulatory Agency (PMRA) is to protect human health and the environment by minimizing the risks associated with the use of pesticides. The fundamental way in which it does this is through the implementation of the Pest Control Products Act (PCPA) and its Regulations which state that all products imported, manufactured, sold or used in Canada to control pests must be registered. In order to be registered a pesticide must meet two criteria. The health and environmental risks must be found to be acceptable and the product must be deemed to have value. This Chapter examines the manner in which the PMRA, through its registration process, determines both the risks and value associated with the use of pesticides. The subsequent Chapter discusses the manner in which PMRA uses regulations to manage risk. Information on generic risk assessment and risk management practices is outlined in Appendix 8.1 and 8.2.
8.2 The risk presented by the use of a pesticide to human and environmental health is a function of two things, the toxicity of the pesticide and the quantity of the pesticide to which humans and the environment are exposed. Accordingly, the PMRA requires both adequate toxicity data and adequate exposure data in order to conduct good risk assessments.115 The registrant (person wishing to register a pesticide) is required, by the PMRA, to perform a number of tests on the toxicity of the pesticide and its fate in the environment. The PMRA analyzes the results to determine whether the pesticide should be registered and how it should be used. Increasingly, the general practices involved in conducting risk assessments are harmonized internationally and the PMRA follows these practices.
8.3 It is important to note that it is the registrants and not the PMRA who conduct the laboratory and field studies upon which the regulatory decisions are based. Some witnesses and Committee members are concerned by this fact, seeing the possibility for biases to enter studies conducted and submitted by the registrants. In order to ensure the quality and integrity of the data submitted, the PMRA requires that the studies be conducted according to what is known as good laboratory practice (GLP) and has published a Regulatory Directive with this title. The principles of GLP are intended to promote the quality and validity of test data. GLP covers the organizational process and the conditions under which laboratory and field studies are planned, conducted, monitored, recorded and reported. The Organization for Economic Co-operation and Development (OECD) has harmonized GLP activities and has established a monitoring scheme via Good Laboratory Practice Monitoring Authorities (GLPMA). A GLPMA audits participating laboratories and issues compliance statements to those that meet the GLP criteria.
8.4 All studies for the PMRA that are initiated after April 1, 2000 must be GLP compliant and will, therefore, require a certificate to this effect. The PMRA may grant waivers to laboratories that do not have GLP certification (via the GLPMA) depending on their certification under similar programs, and the relative significance and potential impact of the studies in question.
8.5 The Committee discussed the possibility of recommending that the PMRA conduct its own risk assessments rather than having them done by the registrant. A recommendation to this effect is not being made at this time because of the existence of the PMRA's GLP program, the feasibility of conducting these types of studies for all products and the fact that other federal departments also accept data submitted from the registrants in support of product registration. We do however think that it is important that the PMRA be more specific about the conditions under which it grants waivers.
8.6 It is clearly impossible to perform toxicity experiments on humans. Tests conducted in support of registration are therefore performed on mammals with relatively short life spans such as rats, mice and dogs, and the results are extrapolated to humans.
8.7 There are a number of potential health effects that pesticides may cause, ranging from no effect to death. The toxicity tests required of the applicants are designed to observe a range of specific effects (often described as endpoints). These tests include:
- acute toxicity;
- short-term toxicity;
- long-term toxicity and carcinogenicity;
- reproductive and developmental toxicity;
- genotoxicity;
- metabolism and toxicokinetics;
- neurotoxicity; and
- immunotoxicity and endocrine disrupting potential (based on the tests listed above).116
8.8 Neurotoxicity tests are normally required only for classes of chemicals known to interfere with the nervous system. Endpoints such as the effects on the immune system and the effects on the hormonal system through endocrine disruption do not currently have specific tests but are examined through the results of other studies. The PMRA publishes regulatory directives, available to registrants and the public, that list the tests required for different product types. According to the PMRA, these lists of tests are becoming increasingly harmonized internationally, especially with the United States.117 In addition, the actual methods for conducting the tests are standard methods published by the OECD118 and/or the United States Environmental Protection Agency.
8.9 The lists of what tests are required for registration must be flexible so that they can be modified to accommodate new potential risks that become evident as knowledge about pesticides increases. Amendments to the Pest Control Products Regulations have been proposed to ensure that the general registration requirements are described as comprehensively as possible.119
8.10 The Committee is satisfied with this type of amendment, yet is still concerned that the PMRA regulatory directives that specify what is required of applicants, are not up to date with the current status of scientific knowledge regarding the damage pesticides can cause to human health. It is already evident that some substances have the potential for causing severe effects on the nervous and endocrine systems, yet specific mandatory testing for these effects is not a requirement for all registrations. The Committee received a number of briefs concerning this shortcoming. The Sierra Club of Canada, the Canadian Environmental Law Association (CELA), the Ontario College of Family Physicians (OCFP) and the Learning Disabilities Association of Canada (LDAC) propose that developmental neurotoxicity testing and tests for endocrine disruption should be standard requirements for all product types. The Committee found the following statement by Ms. Barbara McElgunn of The Learning Disabilities to be interesting in this regard.
| I was interested in reading the testimony from the transcript of previous people who have appeared before this committee, particularly Dr. Claire Franklin's testimony in which she said that all pesticides undergo an extensive pre-market assessment before they are allowed to be sold in Canada. This statement is often heard from PMRA and it may be comforting and soothing to the public but it is not in our view entirely accurate. For example, there are important gaps in the toxicity database for most pesticides, the lack of developmental neurotoxicity test data being one of them. It is of concern to us that developmental neurotoxicity testing is not mentioned in recent PMRA notices concerning impending re-evaluation of organic phosphate pesticides that act via neurotoxic action, nor in the draft document pertaining to harmonization of rules with the EPA to protect children's health. Though in the U.S. this testing gap is a major issue being addressed.120 |
8.11 Virtually all witnesses called for fully transparent risk assessment and risk management processes and several recommended that the process that the PMRA follows should be clearly defined and published.121 In addition, the LDAC, the CELA and the OCFP proposed that the processes be written into legislation or regulation. The Committee is of the opinion that the risk assessment and risk management processes followed by the PMRA should be made public.
| The Committee recommends that all risk assessment and risk management processes which the Pest Management Regulatory Agency uses be clearly defined and published. |
8.12 While the Committee recognizes that the practice the PMRA follows for collecting and utilising human toxicity data is standard, we find that there is much truth in the evidence given by Dr. Bertell's and the LDAC's presentations.122 If tests are not required for a certain pesticide then the possible endpoints studied in those tests can never be part of the risk equation. In order for the PMRA to act in a precautionary manner, the lists of mandatory toxicology studies for the different categories of pesticides must be more inclusive. Dr. Bertell also thinks that risk assessment methodology is limited in scope because it relies on tests that produce a dose response. Other witnesses suggested that the exposure durations specified in the international protocols should be expanded so that fetal and juvenile development can be more adequately assessed.123
8.13 Evaluating the levels to which humans are exposed to a specific pesticide is a complicated task. Exposure may occur, for example, through handling of pesticides by workers, ingesting residues on food or in contaminated water or through the skin after contact with the pesticide. Human exposure is estimated for workers, those who are inadvertently exposed (bystanders), including children, and for the intake of pesticides from trace amounts that remain on food (food residues).
8.14 The worker and bystander exposure (both dietary and non-dietary) to a pesticide is estimated by the PMRA using information on how the pesticide is used, how much is used, and how often. Results of field trials that are conducted for the registrant by third parties show the level of pesticide residue that could appear on food (given the directions for use).
8.15 While relatively detailed information is available from the PMRA with respect to the general requirements for toxicity studies there is little published information on how the PMRA evaluates exposure. A case in point is the exposure assessments that are conducted for children. Many witnesses stated that estimations were conducted for 70-kilogram workers and simply scaled back for children.124 While this may have been the case in the past, in correspondence to the Committee, the PMRA stated that this is not the case now.125
8.16 The PMRA indicated that there is an extensive international database of exposure studies that the PMRA uses to characterize various subpopulations that may be exposed, including children. For children's non-dietary exposure assessment, exposure from all routes and pathways are considered (e.g. dermal through direct or indirect contact with pesticides, non-dietary ingestion through transfer of residues from hand to mouth and inhalation). The special considerations that were discussed in the Chapter on the special vulnerability of children, such as unique activity patterns and physiological characteristics are factored into these assessments. In the absence of specific data, conservative default values are used to obtain the highest estimation of exposure. Internationally accepted standard approaches for deriving estimates of non-dietary exposure for infants and children have been developed and PMRA scientists are participating in and monitoring this activity closely.126 In correspondence to the Committee, Dr. Bertell pointed out that while the PMRA states that they take into account the differential exposure to children, there is no information on how they take into account the fact that children have "developing immune and neurological systems which may not react in the same way as adults."127
8.17 In order to evaluate health risks to humans the estimated exposure levels are compared to the recommended safe levels as established through toxicology tests.
8.18 The first step in determining safe levels for human exposure is to establish, for each toxicology test, the highest level of pesticide for which no adverse effect or endpoint can be observed in the test animal. This is called the no observed adverse effect level (NOAEL). Of the NOAELs from each test, a reference NOAEL is chosen, often the NOAEL that represents the lowest concentration not to have produced an adverse effect.
8.19 Test animals, etc., however, are not humans. The problem then arises as to how to estimate safe levels in humans from the safe levels estimated for test animals in laboratory studies. This extrapolation is done by the PMRA for two different types of exposure, those to workers or bystanders, and for assessing the risks of food ingestion.
8.20 In this type of assessment the level considered safe for the test animals (reference NOAEL) is divided by the estimated exposure to the workers or bystanders to determine the margin of safety (MOS).
Typically, the PMRA considers a MOS of 100 to be acceptable to account for potential variability in response and extrapolations from animals and humans128 (i.e. the estimated exposure should be 100 times smaller than the reference NOAEL).
8.21 Dr. Bertell and the Campaign for Pesticide Reduction criticized this approach because they believe that in order to assess the possible impacts adequately, the results from actual worker, bystander and community exposure studies are required, in addition to the laboratory studies.129 (Mandatory adverse effects monitoring and reporting will be discussed in the next chapter.)
8.22 In order to establish safe dietary consumption levels for humans when extrapolating from test animals, the PMRA divides the reference NOAEL by a safety factor. Specifically, it divides by a safety factor of ten.
8.23 There is another source of variability that stems from the fact that there are normal differences within the human population. In other words, different people may respond differently to the same exposure. Thus the NOAEL is divided by another safety factor of ten. The PMRA therefore calculates the safe level for humans, called the allowable daily intake (ADI), by dividing the NOAEL by a safety factor of one hundred (factor of 10 times factor of 10).
8.24 The concept of safety factors and their applications to the determination of ADIs for pesticides and food additives was established by the World Health Organisation (WHO) in the early 1960s.130 With respect to safety factors, however, the World Wildlife Fund, as well as the Canadian Environmental Law Association and the Ontario College of Family Physicians suggested that the PMRA should follow the example of the United States in terms of developments in child protection.131In the United States, the Food Quality Protection Act (FQPA) requires the Environmental Protection Agency (EPA) to use an additional safety factor of 10 when assessing the risk posed by the presence of a pesticide in the diet of children. This factor can be eliminated only when reliable data is available to demonstrate that the residue will be safe for children. In the US, this additional safety factor is designed to take into account potential pre- and post-natal toxicity and completeness of the data with respect to exposure and toxicity to children.132 Given the vulnerability of children, fetuses and other sectors of the population discussed in Chapters 6 and 7 (Vulnerability of Children and Other Vulnerable Populations), the Committee feels that an increased level of protection from the potential harm of pesticides is required. We think, therefore, that the PMRA should go beyond the US requirements and take the vulnerability of certain sectors of the population into account when doing any kind of risk assessment, not just children and not just for dietary intake.
8.25 The increase in safety factor will be used as a buffer against current uncertainty surrounding the exposure and sensitivity of children and other susceptible populations to pesticides.
8.26 There are instances where small amounts of pesticides, or residues, remain on a food commodity. To ensure that human ingestion of a pesticide from residues does not exceed the safe level as described by the allowable daily intake, the PMRA establishes maximum limits for the levels of pesticide residues that are allowed on food. These limits are called maximum residue limits or MRLs. The Committee learned that the method for establishing MRLs is similar in all developed countries and international organizations. Based on field trials, estimates are made as to how much pesticide is likely to remain on food and how much of the food will likely be consumed. The MRLs for a pesticide are accepted only if the total of the MRLs for all the possible different commodities adds up to less than the acceptable daily intake for that pesticide. The PMRA currently establishes MRLs on a pesticide by pesticide basis and does not take into account the consumption of multiple pesticide residues. MRLs are listed in the tables of the Food and Drugs Act regulations and infractions are monitored by the Canadian Food Inspection Agency (CFIA), an arms length agency under Agriculture and Agri-Food Canada. The CFIA is responsible for all inspection services related to food safety, economics, fraud, trade-related requirements and animal and plant health programs.
8.27 Between 1994 and 1998 there were a total of 44,379 food samples tested by the CFIA. Of these, 805 samples were found to contain residues that exceeded allowable limits and were therefore in violation of the Food and Drugs Act. Violations in domestic products have been increasing while violations on imported products have been staying constant or possibly decreasing.133 If a food product has a residue of a pesticide that does not have a MRL under the Food and Drugs Act regulations then the residue of that pesticide must be below a default value of 0.1 ppm. (See Appendix 8.3)
8.28 In their testimony, several health protection groups, including the Sierra Club of Canada and the Canadian Institute of Child Health, expressed concern about pesticide residues in food. The public and the media share this concern. An article published in May 1999 noted a study showing that the number of residual pesticides detected in fruit and vegetables had doubled since 1994.134 The CFIA suggested that the increase in pesticide residues detected is due to improved scientific methods which make it possible for experts to detect very small quantities of residues. The CFIA stressed the fact that the concentrations detected in the study noted in the article, did not exceed the prescribed limits and thus did not pose any danger to health. The CFIA explained that it is Health Canada's responsibility to determine whether this kind of observation must lead to changes in the MRLs.135
8.29 The Committee remains concerned about food safety despite the fact that the MRLs are not often exceeded. The concern lies in two major areas. Firstly, in determining MRLs, possible sources of pesticide exposure other than through the diet are ignored. These other sources may contribute to the aggregate risks of pesticides exceeding the established safe level for humans. Secondly, many pesticides act in a similar fashion. MRLs, however, continue to be established on a pesticide by pesticide basis, ignoring the cumulative effects of residues from similar pesticides that could also be present on food and in the environment. Assessing pesticides on an individual basis also ignores possible interactions between different pesticides. The Committee was informed that the PMRA plans eventually to include cumulative and aggregate risk in their risk assessments, particularly since the law in the United States now requires these features to be taken into account.136 The Committee, however, shares the concerns expressed by many witnesses including the Canadian Institute of Child Health, the World Wildlife Fund, the Canadian Environmental Law Association and Ontario College of Family Physicians and the Inuit Circumpolar Conference that pesticides are currently assessed individually despite the fact that they are being encountered in the environment in mixtures.137 This can result in a severe underestimation of the risks to which we are being subjected and must be changed immediately.
8.30 Investigations conducted by the CFIA should consider the MRLs of each individual pesticide on foodstuffs, and the total amount of all pesticide found.
8.31 The PMRA conducts risk assessments of environmental health using virtually the same method it uses to examine risks to human health. Safe levels are estimated by laboratory experiments and then compared to estimates of the concentrations that one might expect to find in the environment given the proposed use of the pesticide. The applicant is responsible for completing the studies required by the PMRA, many of which are now harmonized with those of the international community.
8.32 To determine toxicity, a range of species including birds, invertebrates, plants and fish species are subjected to long and short term exposure, and to a variety of concentrations of the pesticide.138 For each test and species the highest concentration at which no effect is observed is recorded. This is the no observed effect concentration (NOEC). The lowest NOEC, recorded for the most sensitive species, is deemed to be a safe environmental concentration.
8.33 The environmental concentration of the pesticide is estimated using a number of analytical results. The pesticide's chemical properties are determined (e.g. physical state, vapour pressure, how easily it can dissolve in water and/or fat). How long the pesticide will stay in the environment before it degrades is determined by studies on its degradation by light, water, air and biological processes. The PMRA also determines whether or not the pesticide may accumulate in organisms higher up the food chain.139 These studies are used to estimate how the pesticide moves in the environment and to estimate the expected environmental concentration. The registrants or government departments may perform field studies to obtain actual environmental measures of a pesticide's behaviour.
8.34 Environmental risk assessment compares the estimated safe concentration for the environment (reference NOEC) with the expected environmental concentration (in much the same way as for worker/bystander assessments). A high ratio between the NOEC for the environment and the expected environmental concentration indicates a large margin of safety (MOS), with limited impact of the pesticide expected. As the ratio decreases the risk that the pesticide will cause harmful effects on non-target organisms increases.
8.35 The Committee has learned that, in some instances, the current environmental assessment fails to prevent environmental damage. As an example, there have been numerous fish kills reported in Prince Edward Island as a result of pesticide runoff following applications made according to the label directions.140 Officials with the Department of Technology and Environment for that province believe that the review process for environmental risk needs to be improved to include greater emphasis on potential environmental impacts and to increase the quality and quantity of data reviewed.141 The Committee feels that increased co-operation with other federal and provincial departments will help with this goal.
8.36 Dr. Bernard Hill, an Agriculture and Agri-Food Canada Environmental Chemist at the Lethbridge Research Centre, gave an example of an instance where he submitted data about a pesticide and never received an adequate response as to why the product was eventually registered. The Committee is surprised that the PMRA seems to be ignoring evidence from experts indicating that there are negative environmental effects resulting from the use of certain pesticides. The testimony from some experts indicates that particular pesticides should be banned from certain uses or de-registered altogether, yet the PMRA is on record as having said that it finds the risk to be minimal.142
Please refer to Chapter 15 for more information on interdepartmental co-operation.
8.37 In order for a pesticide to be registered, the registrants must not only demonstrate that the pesticide does not pose an unacceptable risk to human or environmental health, they must also demonstrate that the pesticide has value. The value, or usefulness, of pesticides can be a controversial topic. For those who believe that pesticides can make positive contributions, the value of a pesticide lies in its contribution to managing pest problems. Pesticide use can have economic, health and environmental benefits both directly and indirectly.
8.38 Economic benefit can be measured as decreased loss of crop yield due to weed invasion or in the decreased loss of wood due to mould in lumberyards. Health benefits can result from situations such as killing cockroaches, using fungicides in carpets to prevent fungal growth during the life of the carpet, or using chlorine to disinfect water in swimming pools. Examples of environmental benefits of pesticide use include the use of chlorine to clean water in wastewater treatment facilities143, the use of fumigants to inhibit the entry of foreign insects that could devastate Canadian forests and the use of insecticides to prevent the destruction of urban forests.
8.39 There are witnesses who believe that the risks far outweigh the benefits for most, if not all, uses of pesticides.144 Certainly, all community-based groups that appeared before the Committee believe that the potential risks of using pesticides for cosmetic purposes far outweigh any benefits. The Committee agrees that the risks arising from cosmetic uses of pesticides far outweigh the benefits and that these uses should be deemed to have no value. Furthermore, the Committee agrees that pesticides that have no value should not be registered. Please refer to the final recommendation in Chapter 12.
8.40 On the other hand, some witnesses agree that pesticides can have positive effects, but are aware that these benefits must be viewed in light of the costs. This not only includes monetary cost but also those associated with negative effects on human health, on other organisms, or on the environment. These costs are difficult to assess since there are few standard ways of measuring them. The PMRA's value assessments have three main components: efficacy, economics, and sustainability.145 Only efficacy and sustainability will be dealt with in this report.
8.41 Sustainability evaluation is a relatively new addition to the consideration of value by the PMRA.146 It is an assessment of the role of the proposed treatment in pest management and the overall production systems for the commodity to be treated, including:
- compatibility with and contribution to sustainable production practices and integrated pest management, including consideration of pest biology and the population level at which an organism becomes a pest;
- comparison with alternative products and practices, including potential contribution to risk reduction (for example, by virtue of lower persistence, toxicity or bioaccumulation, or reduced impact on beneficial and other non-target organisms); and
- contribution to resistance management.147
Integrated pest management and sustainability will be discussed further in Chapter 11 on Alternatives to Pesticides.
8.42 Registrants must perform efficacy studies, normally in the form of field trials to assess product performance and host tolerance. These trials also enable them to establish appropriate label claims and to determine the lowest application rate and frequency required to provide effective and reliable pest control, without damaging the host or crop. The PMRA assesses the results of these studies to determine if the product works as the registrant claims and to recommend the lowest possible application rate that continues to control the pest while minimizing health and environmental risks.148
8.43 Agricultural and industrial stakeholders, through the Economic Management Advisory Committee,149 are recommending that this requirement be greatly reduced or eliminated in an effort to reduce timelines.150 The Committee feels that this would be a mistake. Efficacy analysis is a valuable aid in minimizing the application of pesticides and associated risks to the environment and human health.
| The Committee recommends that the Pest Management Regulatory Agency continue to require a full set of efficacy data for the registration of pesticides. |
Additional Risk Assessment Practices
8.44 Most of the toxicity data for a pesticide product is for the ingredients that make the pesticide work (active ingredients). Formulants are the ingredients in the end use product, other than the active ingredients and often make up the bulk of the products. They are considered trade secrets and do not have to be disclosed on the label. Often called "inert ingredients," many formulants are not inert at all. While some may be true inert ingredients, they may also be solvents, surfactants (soaps) or oils that may have toxic properties.151 Formulants are only starting to become an important part of the safety assessment of pesticides.152 The Canadian Environmental Law Association and the Ontario College of Family Physicians proposed that the PMRA complete the development of its policy on formulants. This policy would outline how the PMRA will go about completing assessments of formulants in registered pesticides. These formulants should be assessed such that their use would not be allowed until their potential effects are understood.153 A PMRA policy on formulants has been drafted and is expected to be published for comment in 2000.154
8.45 Contaminants are present in products but, unlike formulants, are not intentionally added. Contaminants are normally found at very low concentrations and are thus often called microcontaminants. Many contaminants do not pose any safety risk (e.g. water) but, as with formulants, some can be of significant concern.
8.46 Given the potentially toxic nature of formulants and contaminants (including microcontaminants) in a pesticide, the Committee is concerned that their possible environmental and health risks are not being adequately assessed. (Please refer to Chapter 13 on Informing and Involving the Canadian Public for information on the labelling of 'toxic' ingredients.)
8.47 Normally, major new uses of pesticides require an assessment by the PMRA. Currently, if a pesticide is registered for use on corn and is subsequently going to be used on a genetically modified corn, the PMRA does not re-assess the pesticide. The Canadian Food Inspection Agency (CFIA) informed the Committee that they do not consider changes in pesticide use patterns during the course of their assessment of genetically engineered plants. The PMRA only provides advice on resistance management to CFIA during the registration process for genetically modified plants. The Committee is concerned that this type of change in use pattern can be effected without a re-evaluation of that pesticide by the PMRA.
8.48 Pesticide manufacturers are ultimately responsible for the safety of their products according to the PMRA's testimony of June 1, 1999.155 For pesticides that have yet to be registered the burden of proof lies with the manufacturer of the pesticide to demonstrate that the pesticide is safe for its intended use. Within the current regulatory system this burden shifts slightly to the government and the general public for pesticides once they are already registered and on the market. According to The Canadian Environmental Defence Fund, once a pesticide is on the market, the burden of proof that any unacceptable risk does occur rests with those who seek the removal of the product from the market.156 While the Committee recognises that the government must accept a certain amount of responsibility when registering products, we believe that the current system puts too much of the onus on the public and government and not enough on the manufacturer.
| The Committee recommends that the burden of proof that a pesticide does not pose an unacceptable risk remain with the manufacturer both before and after registration. |
APPENDIX 8.1: INTEGRATED RISK ANALYSIS*
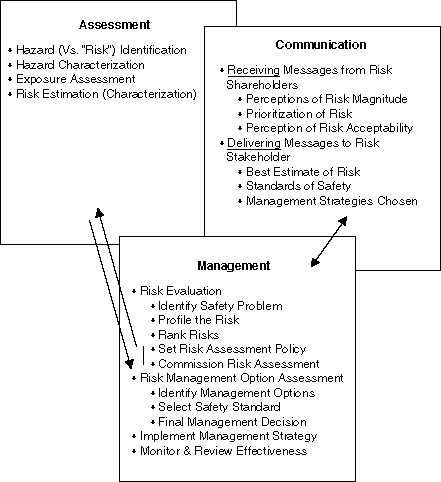
* Conrad Brunk, Risk Management Workshop, 1998.

The basic steps of risk assessments are Hazard Identification, Exposure Assessment, Consequence Assessment and Risk Estimation.
- Hazard Identification: An assessment is begun with an attempt to identify the hazards associated with the pesticide. This is where the actual properties of the substance in question are examined as well asits propensity for causing toxic effects. This is also called the inherent toxicity of the substance. The hazard posed by a substance can be measured or quantified by determining the amount that kills 50% of the test organisms (LD50), the amount that causes an effect in 50% of terrestrial organisms (EC50), the amount that causes an effect in 50% of aquatic organisms, etc. Governments have guidelines that classify these numbers based on relative toxicity so that a comparison between substances is possible when evaluating the potential hazards to different organisms (e.g. rat, fish, bird) in different media (i.e. air, water, soil and sediment). These classification guides are very useful in step three and are consequently becoming increasingly harmonized internationally as a result of international protocols and the work of organizations such as the Organization for Economic and Co-operation and Development (OECD).
- Exposure Assessment: This step determines if the substance enters the environment or comes in contact with humans or animals. It is here that questions are asked, such as when, where and how does exposure take place? In what quantities/concentrations? Is the substance persistent and will it bioaccumulate thus tending to continue to increase in concentration in the environment?
- Consequence Assessment: The first and second steps are fitted
together to form the basis of risk. Hazard times Exposure is equal to Risk. If either
Hazard or Exposure is missing, or equal to zero, then there is no risk (3 X 0 = 0).
Hazard X Exposure = Risk
With this equation it becomes clear that there can be a substance with lower inherent toxicity (and therefore Hazard) but high Exposure that results in high risks (2 X 6 = 12) or a substance with higher inherent toxicity and lower Exposure that also results in high risk (4 X 3 = 12).
Consequence Assessment involves identifying the outcome of the risk. It is where guidelines are used and an attempt is made to determine what would be the actual effect on the person/organism/environment/etc. Is the impact major (e.g. death, loss of reproductive capacity), minor (e.g. slight decrease in toenail size), or moderate. It is also in this step that what and/or who is the most at risk is identified. - Risk Estimation: This last step involves determining the acceptable level of exposure to the target populations or, in other words, how much can humans, fish, frogs, children, etc. be exposed to the substance with no short or long term adverse effects. The No Observable Adverse Effect Level (NOAEL) is most often used to calculate the estimated acceptable exposure. In conducting a risk assessment the NOAEL is divided by a safety factor.
Once an acceptable dose is established (NOAEL / safety factor) it is compared to what has been estimated from the exposure assessment. If the predicted exposure is higher than the calculated acceptable dose then controls must be put in place to mitigate release and/or exposure. This enters into risk management.
APPENDIX 8.3: A Summary of Canadian Food Inspection Agency's Report on Levels and Incidences of Pesticide Residues in Selected Agricultural Food Commodities Available in Canada During 1994-1998
This document summarizes the information contained in the Canadian
Food Inspection Agency's report on the Levels and Incidences of Pesticide Residues in
Selected Agricultural Food Commodities Available in Canada During 1994-1998, dated 6
November 1998. Results for the fiscal year
1998-1999 have not yet been published.
For reporting purpose, food commodities are divided into four categories, domestic fresh fruits and vegetables, domestic processed fruits and vegetables, imported fresh fruits and vegetables, and imported processed fruits and vegetables. There are four basic figures that are reported in this document:
- the total number of samples taken;
- the number of samples that were without detectable residues (Green Light);
- the number of samples that had positive results for residues (Yellow Light); and
- the number of those positives that were in violation of the maximum residue limits set by Health Canada (Red Light).
There were a total of 44,379 samples taken during this reporting period. Their breakdown between the four categories of food commodities is as follows:
Table 1: Total Number of Sample Taken
Total |
Fresh |
Processed |
Domestic |
6,879 |
378 |
Of the total 44,379 samples taken there were 35,487* samples that were without detectable residues (W.D.R.). Their breakdown between the four categories of food commodities is as follows:
Table 2: Total Number of Samples
without
Detectable Residues (W.D.R.)
Total |
Fresh |
Processed |
Domestic |
5,511
(80%) |
349
(92%) |
Of the total 44,379 samples taken there were 10,682* samples that were found to contain residues (positives). Their breakdown between the four categories of food commodities is as follows:
Table 3: Total Number of Samples
that
Tested Positive for Residues
Total |
Fresh |
Processed |
Domestic |
1,710 (24.8%) |
33 (8.7%) |
Of the total 44,379 samples taken there were 805* samples that were found to contain residues that exceeded allowable limits and were therefore in violation of the Food and Drugs Act. Their breakdown between the four categories of food commodities is as follows:
Table 4: Total Number of Samples in Violation
Total |
Fresh |
Processed |
Domestic |
82 (1.2%) |
2 (0.53%) |
Generally speaking violations in domestic products have been increasing (0.4% prior to 1991, 0.55% from 1992-1994 and 1.2% for 1994-1998) and violations on imported products has been staying constant or possibly decreasing (2.6% prior to 1991, 2.74% from 1992-1994 and 1.94% for 1994-1998).
The four appendices in CFIA's report show the findings for the different commodities sampled and the residues found by pesticide. These appendices are for each of the four categories. There are different standards for the different pesticides and there are different concentrations for different food commodities. This is so that a food that makes up a larger portion of the diet has stricter standards. Specific concentrations can be found in the Regulations under the Food and Drugs Act.
Specific residue finding continue to show that the occurrence of environmentally harmful organochlorines such as DDT and metabolites, aldrin, dieldrin, endrin, heptachlor, lindane and a few others are rarely found in domestic samples. In imported shipments these compounds, especially DDE (the major metabolites from DDT breakdown) continue to be found in greater frequencies. Even in imported shipments though, these pesticides rarely exceed prescribed levels.
115 R. Bertell, Correspondence to the Committee, February 27, 2000; Learning Disabilities Association of Canada (LDAC), Correspondence to the Committee, March 2, 2000; Canadian Institute of Child Health (CICH), Correspondence to the Committee, March 3, 2000.
116 Pest Management Regulatory Agency, Web site, December 1999.
117 Evidence, Meeting No. 2, November 2, 1999.
118 Organization for Economic Co-operation and Development (OECD), Guidelines for the Testing of Chemicals, October 1998.
119 Pest Management Regulatory Agency (PMRA), Proposed Amendments to the Pest Control Products Act, January 1999.
120 Evidence, Meeting No. 4, November 16, 1999.
121 Canadian Association of Physicians for the Environment, Brief to the Committee, 1999; the Canadian Environmental Law Association and the Ontario College of Family Physicians, Brief to the Committee, 1999; and by the Crop Protection Institute of Canada, Brief to the Committee, 1999.
122 Evidence, Meeting No. 16, December 14, 1999; Evidence, Meeting No. 4, November 16, 1999.
123 Correspondence from Learning Disabilities Association of Canada to the Committee, March 2, 2000; Correspondence from the Canadian Institute of Child Health to the Committee, March 3, 2000.
124 Canadian Association of Physicians for the Environment, Brief to the Committee, 1999; the Canadian Environmental Law Association and the Ontario College of Family Physicians, Brief to the Committee, 1999; and the Crop Protection Institute of Canada, Brief to the Committee, 1999.
125 Correspondence from Dr. C. Franklin to the Committee, February 29, 2000; August 24, 1999 and December 20, 1999.
126 Correspondence from Dr. C. Franklin to the Committee, February 29, 2000.
127 Correspondence from Dr. Bertell to the Committee, February 27, 2000.
128 Pest Management Regulatory Agency, Web site, December 1999.
129 Evidence, Meeting No. 16, December 14, 1999; Evidence, Meeting No. 3, November 4, 1999.
130 Amdur, M.O., Doull, J., and Klaassen, C.D, Casarett and Doull's Toxicology: the Basic Science of Poisons, Fourth Edition, Pergamon Press, 1991.
131 Evidence, Meeting No. 5, November 17, 1999; Evidence, Meeting No. 11, December 1999.
132 United Stated Code, Title 21, Chapter 9, section 346a.
133 Canadian Food Inspection Agency, "Levels and Incidences of Pesticide Residues in Selected Agricultural Food Commodities Available in Canada During 1994-1998," Technical Report, November 1998.
134 "Level of Pesticide Residues up in Canadian Produce," Globe and Mail, May 24, 1999.
135 Evidence, Meeting No. 128, June 8, 1999.
136 Evidence, Meeting No. 126, June 1, 1999.
137 Evidence, Meeting No. 4, November 16, 1999; Evidence, Meeting No. 5, November 17, 1999; Evidence, Meeting No. 16, December 14, 1999; Canadian Environmental Law Association and Ontario College of Family Physicians, Brief to the Committee.
138 Pest Management Regulatory Agency, Web site, December 1999.
139 Ibid.
140 Evidence, Meeting No. 14, December 8, 1999.
141 Ibid.
142 Evidence, Meeting No. 12, December 2, 1999.
143 Canadian Water and Waste Water Association, Brief to the Committee.
144 Evidence, Meeting No. 13, December 7, 1999; Evidence, Meeting No. 15, December 13, 1999.
145 Pest Management Regulatory Agency, Web site, December 1999.
146 Ibid.
147 Ibid.
148 Ibid.
149 This Committee was formed in 1995 to advise the PMRA.
150 Pest Management Regulatory Agency, Web site, EMAC Workplan, Goal 2.1.3, March 10, 1999.
151 World Wildlife Fund, The Problem with Pesticides in Canada: A Briefing Book for Parliamentarians, April 1999; Sierra Club of Canada, Brief to the Committee.
152 Pest Management Regulatory Agency, Web site, December 1999.
153 Canadian Environmental Law Association and the Ontario College of Family Physicians, Brief to the Committee.
154 Evidence, Meeting No. 23, February 17, 2000.
155 Evidence, Meeting No. 126, June 1, 1999.
156 Evidence, Meeting No. 5, November 17, 1999.