HEAL Committee Report
If you have any questions or comments regarding the accessibility of this publication, please contact us at accessible@parl.gc.ca.
PART I: CONTEXT
The formal work for the House of Commons Standing Committee's study on natural health products (NHPs) began when it agreed to the terms of reference suggested in a letter from the Minister of Health, the Honourable Allan Rock, on November 13, 1997.
TERMS OF REFERENCE FOR THE COMMITTEE'S STUDY
|
|
That the Standing Committee on Health consult, analyze and make recommendations regarding the legislative and regulatory regime governing traditional medicines (including, but not limited to, traditional herbal remedies, traditional Chinese, Ayurvedic and Native North American medicines), homeopathic preparations and vitamin and mineral supplements; That the Standing Committee consult broadly with stakeholders, including, but not limited to, associations and individuals representing consumers, manufacturers, distributors, growers, importers, exporters and retailers; That the Standing Committee consider the objectives of providing consumers freedom of choice and access to natural health products while ensuring the quality and safety of such products; That the Standing Committee consider the legislative and regulatory regimes governing natural health products in other countries. |
In the late fall of 1997, the Committee received extensive background information from the two major divisions of the Health Protection Branch at Health Canada involved in the regulation of NHPs, the Therapeutic Products Program (TPP) and the Food Directorate. The Advisory Panel on Natural Health Products (APNHP), established in May 1997 by the Minister of Health, presented its interim report to the Committee in February 1998 and followed with a final report in May. The meetings with the broader public that began in February 1998 continued until May 1998. In addition to extensive hearings in Ottawa, our work as a Committee included videoconferencing from Calgary, Halifax and Winnipeg and travel to Montreal, Toronto and Vancouver. Overall, we received testimony from a broad range of Canadian consumer, practitioner, industry and regulatory representatives. As well, we heard from international participants from Australia, Germany, the United Kingdom and the United States.
The members of the Committee wish to thank all those who devoted their time and efforts to the Committee's study-over 300 individuals in appearances before the Committee and over 1000 individuals through letters and briefs.
C. Background
From the beginning of its study, Committee members were aware of the intensity of the debate over the regulation of NHPs. We also had knowledge of various actions taken by the federal government over the last decade that had overtly increased public awareness and discussion. These actions included Schedule 705 with its proposed list for restriction of herbs and botanicals as foods; the enactment of the Controlled Drugs and Substances Act; work by committees of the U.N. Codex Alimentarius Commission; and cost recovery efforts undertaken by Health Canada in the middle of the 1990s. The Advisory Panel on Herbal Remedies (later renamed Advisory Panel on Natural Health Products), established in May 1997 to advise on the development of an appropriate regulatory framework, provided a parallel forum as the Committee commenced its work.
Over the months, the Committee heard about multiple forces creating pressure for regulatory changes affecting NHPs. As a backdrop, witnesses noted that the current Food and Drugs Act was enacted in 1952 and, although the Act itself contains only 20 pages, over 500 pages have been added to the accompanying regulations without being subject to any full parliamentary procedure or scrutiny. The results of a CTV/Angus Reid Group Poll in August 1997 indicated that 67% of Canadians feel that the federal government should regulate NHPs to ensure product safety and quality, the Committee heard repeatedly that a major reassessment is long overdue.
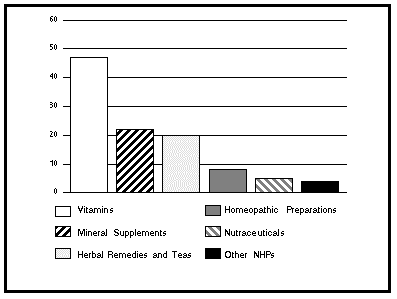
One pressure facing regulators emanates from the tremendous interest in and demand for NHPs and the need to accommodate both the industry and consumers in this area. Canadians are taking more responsibility for their own health and, in many cases, this has led to an increased awareness about NHPs. A survey by the Canada Health Monitor (Survey 16, June-July 97) revealed that 56% of Canadians reported taking one or more NHPs in the past six months. Among them, 47% had taken vitamins, 22% mineral supplements, 20% herbal remedies and teas, 8% homeopathic products, 5% nutraceuticals and 4% some other products.
On the industry side, distribution channels providing access for consumers include health food stores, supplement and nutrition stores, NHP practitioners, pharmacies and drug stores, grocery stores, multilevel or network marketing; and mail, phone and Internet marketing. The extremely diverse nature of both the manufacturing and distribution sectors of this industry makes reliable data on the total market size of NHPs difficult to ascertain. However, estimates provided to the Committee suggest that the gross income of the NHP industry in Canada ranged between 1.5 and 2 billion dollars in 1997, with annual growth of 10 to 15%. The Committee was told that, while there are a few sizeable NHP manufacturing companies, the NHP industry is mostly made up of small and medium sized companies that are labour intensive. It was also indicated that the commercial growing of herbs represents a viable alternative for Canadian farmers.
FIGURE 1
CONSUMPTION OF NATURAL HEALTH PRODUCTS
IN CANADA, 1997
(Percentage of Users per Category of Products)
Committee members also became increasingly aware of international pressures for harmonization of standards. This means that NHP regulators must consider the advisability of accepting common protocols and formats for testing, data submission, inspection as well as mutual recognition of another regulatory body's decisions. Proximity to the United States, where unlike most other developed countries, NHPs are generally regulated as foods (dietary supplements), has created particular problems for Canadian regulators, as well as the industries, practitioners, and consumers involved with NHPs.
The Committee recognizes that our mandate on NHPs covers many types of products and affects many different stakeholders. We acknowledge both the complexities of the subject and the requirements for immediate action. In the following chapters, we provide our version of a framework for regulatory and policy initiatives that can be implemented by Health Canada over a short period in partnership with other interested parties. To facilitate progress, we have recommended in our final chapter that a transition team be established immediately to begin the movement toward a new process for NHPs.
Analyzing various legislative, regulatory and policy options governing NHPs and evaluating their possible consequences was not an easy task for us as Committee members. In particular, we had to thoroughly review and understand the Food and Drugs Act and its Regulations, and assess the appropriateness of their application to NHPs. But foremost, we had to enhance our personal knowledge of NHPs. Although many of us are consumers of NHPs, either regularly or occasionally, we had no pretensions of being experts in the field of NHPs.
For all of us, a primary objective of any new regulatory framework for NHPs must take into account the well-being of consumers. On the whole, our desired outcome is a regulatory framework for NHPs that (1) protects the health of consumers (2) respects consumers' access to products and (3) guarantees product safety and quality.
All members of the Committee share the same objective - that the health of Canadians must remain as the most vital criterion underlying any regulatory analysis.
The work of the Committee was complicated by the fact that, although many witnesses shared similar views with respect to the regulation of NHPs, others expressed conflicting opinions. For this reason, we decided to establish a list of principles to steer us in our decisions and recommendations. This list of guiding principles was adopted unanimously by Committee members and includes:
Nature of NHPs: NHPs are different in nature from and must not be treated strictly as either food or pharmaceutical products.
Safety: Safety of NHPs is of primary concern.
Quality: The NHP industry must meet clearly defined and established standards of quality.
Access: NHP regulations must not unduly restrict access by consumers.
Informed Choice: NHP consumers must be provided with pertinent information about the products they purchase.
Cost: NHP regulations must not place inappropriate cost on industry, consumers and government.
Decision-Making: Decision-making power must be given to a regulatory body with expertise and experience with NHPs.
Availability of Appeal: An open and transparent process of appeal must be available to NHP stakeholders.
Transparency: Information regarding decisions and the regulatory system must be readily available to NHP stakeholders.
Cultural Diversity: NHP regulations must respect diverse cultural traditions.
The Committee's terms of reference cover three major types of NHPs: traditional medicines (including Chinese, Ayurvedic, and North American Aboriginal herbal medicines); homeopathic preparations; and vitamin and mineral supplements. The Food and Drugs Act and its accompanying Regulations govern the sale of these products in Canada. Although the Act does not define the term "natural health products," it does contain related definitions. Under the Act, NHPs can be either foods or drugs.
If NHPs are a food product, they are governed specifically by sections 4 to 7 of the Act and Part B (titled Foods) of the Regulations. The Act provides the following definition of food:
|
Includes any article manufactured, sold or represented for use as food or drink for human beings, chewing gum, and any ingredient that may be mixed with food for any purpose whatever. |
NHPs sold as a drug product are governed specifically by sections 8 to 15 of the Act and Part C (titled Drugs) and Part D (titled Vitamins, Minerals and Amino Acids) of the Regulations. The Act contains the following definition of drug:
|
Includes any substance or mixture of substances manufactured, sold or represented for use in (a) the diagnosis, treatment, mitigation or prevention of a disease, disorder, abnormal physical state, or the symptoms thereof, in man or animal, (b) restoring, correcting or modifying organic functions in man or animal, or (c) disinfection in premises in which food is manufactured, prepared or kept. |
Currently, homeopathic preparations and vitamin and mineral supplements are sold as drugs. Some herbal products are sold as foods, while others are marketed as drugs (certain plants are even controlled under the Controlled Drugs and Substances Act). Health Canada's Information Letter No. 771 on traditional herbal medicines, issued to all manufacturers and importers, indicates how the department determines whether a herbal product is a food or a drug:
The most important factors in determining whether a herbal product should be considered a food or a drug are: the pharmacological activity of the ingredients; the purpose for which the product is intended; and the representations made regarding its use, including directions for use. Other factors, such as precise dosage form, may be considered in the determination of a product status. Herbal ingredients appropriate as foods are those that can be consumed more or less as desired due to the absence of pharmacological properties.1
For some witnesses, mainly those representing consumers, the current definition of food encompasses NHP products, as these, in their view, are "foodstuffs" or "nutritional supplements." By contrast, for some others, NHPs are adequately represented under the definition of drug since, they argued, NHPs are being used to address health and wellness concerns.
Most witnesses, however, contended that neither the definitions for foods nor for drugs properly describe NHPs and that, in fact, these products fall into a grey area between foods and drugs. Some described NHP products as a subset of the food category. Others defined NHPs as a subset of the drug category. For some, NHPs needed to be distinct from both the food and drug categories. These witnesses recommended that a "new" or "separate" or "third" category of products be established for NHPs.
Touching on the contention that NHPs are not adequately described as either foods or drugs, the APNHP recommended that the term "drug product" in the Food and Drugs Act be renamed "therapeutic product" and that this category be divided into two classes - NHPs and pharmaceutical products. The resulting APNHP definition of NHPs was echoed by other witnesses who similarly emphasised the three main components of NHPs as: substances found in nature; presented in dosage form as capsules, tablets, etc.; and with properties for health maintenance and improvement, as well as for disease prevention and treatment. The APNHP definition described NHPs as:
substances or combinations of substances consisting of molecules and elements found in nature, and homeopathic preparations, sold in dosage form for the purpose of maintaining or improving health and treating or preventing diseases/conditions.2
For some other witnesses, NHPs could be classified as either functional foods or nutraceuticals. The Food and Drugs Act does not define these terms, but according to a document by Health Canada:
A functional food is similar in appearance to or may be a conventional food, is consumed as part of a usual diet, and is demonstrated to have physiological benefits and/or reduce the risk of chronic disease beyond basic nutritional functions.
A nutraceutical is a product isolated or purified from foods and generally sold in medicinal forms not usually associated with food and demonstrated to have a physiological benefit or provide protection against chronic disease.3
The members of the Committee acknowledge that the current definitions of a food and of a drug in the Food and Drugs Act do not adequately accommodate NHPs. This is reflective of the Committee's guiding principle on the different nature of NHPs. They also recognize that, when sold in various dosage forms, NHPs do have the potential for therapeutic effects. As a result, it is important to stress that NHPs sold in dosage form should comply with appropriate regulations.
The Committee also wants to note that witnesses did not reach a consensus on whether bulk herbs should be included in the NHP category (since they are not sold in dosage form). Further, witnesses did not agree on the various types of products (vitamins, minerals, amino acids, co-enzymes, etc.) that could be included in the NHP category.
Overall, the Committee does not feel it is appropriate to provide an explicit list of products or a specific definition for NHPs as members feel that they do not have the adequate expertise to undertake these tasks. Health Canada will work with a newly created and separate NHP Expert Advisory Committee to establish the new regulatory framework for NHPs. This new NHP Expert Advisory Committee, in conjunction with Health Canada, will determine whether the NHP class should include functional foods, nutraceuticals, etc. and will set out an appropriate definition. Specific recommendations about the Expert Advisory Committee are contained in the following chapter.
Therefore, the Committee recommends that:
Health Canada, in conjunction with a new separate NHP Expert Advisory Committee, set out an appropriate definition of NHPs and amend the Food and Drugs Act accordingly;
Health Canada, in conjunction with the new NHP Expert Advisory Committee, examine the status of bulk herbs for legislative purposes.
1 Health Canada, Information Letter No. 771, January 5, 1990, p. 1-2.
2 Advisory Panel on Natural Health Products, Regulatory Framework for Natural Health Products, Final Report, May 13, 1998, p. 6.
3 Health Canada, Draft Policy Option Analysis: Nutraceuticals/Functional Foods, October 9, 1997, p. 11, footnote.