HEAL Committee Report
If you have any questions or comments regarding the accessibility of this publication, please contact us at accessible@parl.gc.ca.
chapter 2
overview
The Committee heard considerable evidence during the public hearings about the current situation in relation to organ*and tissue donation and transplantation nationally and internationally. It learned about the profile of donors and recipients, about the numerous organs and tissues that must be considered, about the multiple organizations, facilities, and healthcare professionals, and about the diverse roles of provincial governments. All of this was crucial to the Committee's primary goal of understanding the appropriate role for the federal government in this area. The following sections provide an overview of the donor and recipient profile, the key organs and tissues as well as the primary governmental, non-governmental and international participants pertinent to understanding the existing status of donation and transplantation in Canada.
The Committee was aware from its inception that the most significant players in the donation and transplantation process are the donors and the recipients. Members listened carefully to the personal stories of individuals and families, which contributed greatly to the understanding of the issue. Current data suggests the following about organ donors and recipients; little is known about tissue donors and recipients.
A brief profile of cadaveric donors (438 in 1996) suggests that most were multi-organ donors (79.1% in 1996). Of the single organs, kidneys were the most frequently donated, followed by livers, hearts and lungs. The most common cause of death for organ donors (48%) is an intracranial event such as a cerebral bleed. Traumas such as motor vehicle accidents or gunshot wounds account for approximately 33% of donor deaths. The average age of a cadaveric donor is between 34-38 years.
The most common transplant recipient is a middle-aged Caucasian male. Of recipients, 87% are between 18 and 64 years of age and 65% are male. Males constitute 84% of heart transplant recipients.
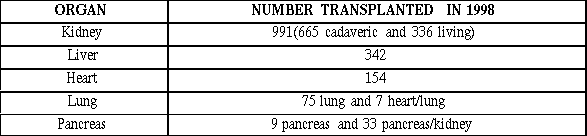
Witnesses spoke about the many organs and tissues that can be donated and used for transplantation. Organs that can be transplanted include kidney, liver, pancreas, heart, lung, and bowel. Tissues include blood, cornea, bone marrow (as a source of stem cells), stem cells, cord blood (as a source of stem cells), islets, skin, sperm and eggs. The processes for procurement, storage and transplantation of these organs and tissues vary. The following sections provide additional information on the number of organ and tissue transplants, sources of donated organs, and procured tissue banks.
The greatest transplant activity involves kidneys, mostly as a result of diabetes and congenital defects. Kidney failure is unique among organ failures in that dialysis can sustain patients awaiting transplant; no such therapy exists for the remaining vital organs. Bowel or intestinal transplantation, still considered experimental, is used for patients who are unable to absorb adequate nutrients or who have poor bowel motility.
The tissues used for transplant and their donation requirements are generally less well known and understood by the public than is the case with organs. Some of the commonly transplanted tissues include blood, skin, cornea, semen, and bone marrow.
Blood is the most frequently transplanted or transfused tissue and as such it is the most publicly recognized. If the cornea, a form of transparent skin similar to a contact lens, becomes opaque, causing blindness, transplantation is the only treatment. Skin is frequently required for grafting, particularly in the case of burn victims. Whenever possible autografts are performed but for extensive burns allografts may be required.
Bone marrow, stem cells and cord blood are usually considered together because stem cells are the identified component of both bone marrow and cord blood that permits successful transplantation of these tissues. The stem cells are responsible for the production of all blood cells; including the red cells that carry oxygen, the white cells that fight infection, and the platelets that are required for clotting. They are often used to correct disorders such as aplastic anemia and leukemia. Where bone marrow is renewable, cord blood has the benefit of making use of an otherwise discarded material. Islets are the cells of the pancreas responsible for the production and secretion of insulin. It is hoped that islet transplantation will ultimately be the treatment for diabetes.
Reproductive tissues include semen or sperm, ova, zygotes and embryos. Sperm and ova (eggs) can be collected from individuals for use in their own assisted fertilization or for donation to others for reproductive purposes.
3. Sources for Donated Human Organs and Tissues
There are two categories of human donors: cadaveric and living. Cadaveric donors constitute the most commonly known form for both organs and tissues. Organ transplants in particular rely on donations that occur after the donor's death. The principal category of cadaveric donation is the brain dead donor; with the non-heart beating donor constituting a lesser source.
Living donors for organs include: bilateral organ donors, segmental organ donors and domino organ donors. Bilateral organ donation refers to situations when an individual's second kidney is procured and transplanted into a blood relative and, in some countries, an emotionally related person, such as a husband or wife. Segmental donation refers to the removal of a portion of an organ from a living, usually related, donor. Currently, this is most common with liver but investigations suggest the use of segmental transplants of lung, pancreas and small bowel. The domino donation refers to the possibility of a live heart donor where the recipient of a combined heart/lung transplant in turn donates his or her heart for transplantation.
Tissues can also be donated from living donors. Reproductive tissues such as semen/sperm are frequently used for artificial insemination. Bone, cartilage, tendons, heart valves, and nerves can be donated during surgery when such tissues are to be removed for treatment and would otherwise be discarded.
Once a tissue or organ has been removed from the body it has also lost the blood supply that is its source of nutrition and oxygenation. Without some sort of intervention the tissue or organ will die. The length of time that an organ or tissue can remain viable varies, depending on the tissue or organ in question and to some extent the age of the donor. Preservation attempts to slow down or suspend this degenerative process. The more vascularized an organ or tissue, the more difficult the preservation. Tissues require less blood supply than organs and therefore can be more safely preserved, frozen and stored for future use. Tissues can be categorized as either regenerative or non-regenerative. The regenerative bodily substances that can be stored, or banked, routinely are blood, skin, bone marrow, sperm and ova. Non-regenerative tissues such as heart valves, veins, tendons, nerves, bones and cornea can also be stored.
As yet banks do not exist for organs and their long-term storage and banking seem unlikely in the near future. Even if the organ shortage was overcome and a surplus achieved, organs require constant perfusion with blood to prevent their deterioration. Preservation techniques for the heart, lung, liver, and pancreas have not been so extensively studied as those for the kidney. The principles of preservation are the same, although these other organs will not tolerate such long periods without a blood supply. Transplantation is performed as quickly as possible, preferably within eight hours for the liver and pancreas, four hours for the heart, and two hours for the combined heart/lung. Much research will be necessary before it is possible to keep organs banked in the same way as blood.
C. LOCAL AND PROVINCIAL PARTICIPANTS
Presently, Canada has multiple local and provincial organizations, facilities, professionals, and governments involved in organ and tissue donation and transplantation. The following section provides background information on their activities. While not inclusive of details on all those involved in this area, it provides an overview of current initiatives.
1. Awareness/Support Organizations
Numerous non-profit awareness organizations, including the Kidney Foundation, the Parkinson's Foundation, the Canadian National Institute for the Blind, the Canadian Liver Foundation, La Fondation Diane Hébert, and the Canadian Cystic Fibrosis Foundation, provided material to the Committee. Most of these organizations are volunteer based, focus on the health and quality of life of their clients, and provide disease-specific support and information services. Most also fund research pertinent to their respective areas. Some like the Association des greffés du Québec establish special services such as the Montreal shelter to support individuals waiting for transplants.
For the most part, these organizations do not view promotion of organ donation as their primary function; however, some have devoted significant time and money to public awareness and education campaigns on this subject. The Mutual Group, an insurance company, has worked with the Kidney Foundation and others to promote awareness. Since 1992, it has committed financial investments up to $3.5 million and provided services from more than 3,000 staff and sales personnel.
Organizations that oversee the procurement and allocation of organs and tissues play a significant role. With a few exceptions like blood and bone marrow, tissue procurement and storage banks continue to be local initiatives, usually independent or hospital based. Some of the key players in tissue procurement who testified before the Committee included: Canadian Blood Services, the Bruce Denniston Bone Marrow Society, the Eye Bank of Canada, the Unrelated Bone Marrow Donor Registry, the Alberta Cord Blood Bank and the Queen Elizabeth II Tissue Bank.
Organ Procurement Organizations (OPOs) are more visible and, although most grew out of local initiatives, are now frequently supported by provincial governments. OPOs provide the link between organ donors and the patients waiting for transplants, with the aim of achieving equitable organ allocation. Their responsibilities are multi-faceted and include: evaluating all possible donor situations, obtaining consent from family members for organ donation, managing a suitable donor's physical state, arranging for blood and tissue typing to test recipient compatibility, ensuring allocation of organs, coordinating surgical transplant teams and ensuring a trouble-free recovery process. A key role for organ recovery coordinators involves education of both healthcare professionals and public audiences about the benefits of organ donation and transplantation. The following information on the structure, funding, and functions of OPOs was provided during their presentations to the Committee.
The British Columbia Transplant Society, established in 1986, has an integrated delivery system for organ transplant services. It has a mandate to coordinate, fund and monitor all solid organ transplant services in British Columbia. This includes the establishment of quality standards, pre-transplant assessment, transplant surgery, follow-up care and the distribution of immunosuppressant drugs. The Society also conducts research and delivers professional and public education. Follow-up services are offered in three Vancouver transplant centres and eight ambulatory community clinics located in Victoria, Kelowna, Kamloops, Surrey, Langley, Trail, Penticton, and Prince George. It is fully funded by the Ministry of Health with an operating budget in 1998/99 of almost $23 million.
The Human Organ Procurement and Exchange (HOPE) of Alberta consists of two HOPE programs that function independently: one is situated in the Foothills Provincial Hospital in Calgary and the other in the University of Alberta Hospital in Edmonton.
The Saskatchewan Transplant Program, a provincial coordinating agency for vital organ donation and heart valve donation, is also linked with the provincial Eye Bank. It operates out of Saskatoon (Royal University Hospital) where kidney transplants are carried out. All other solid organs, which are procured, are offered first to Alberta and Manitoba. The program provides both professional and public education.
The Manitoba Transplant Program, situated in the Health Sciences Centre in Winnipeg, coordinates services for the province. There are two donor/trauma coordinators. Manitoba transplants lungs and kidneys and exports all other procured organs to other provinces or to the United States.
Ontario's Multiple Organ Retrieval and Exchange Program (MORE) was established in 1988 through a joint effort of the provincial government, Ontario Hospital Association, and Ontario Medical Association. Its key role was to keep a province-based electronic listing of patients waiting for an organ in Ontario. The current mandate includes the creation of educational and organ donor awareness campaigns for health professionals and the public, provincial coordination and equitable access. Funded by the government, MORE has a 12-member board of directors composed of representatives from the government, the transplant community, and the public. Organ donor coordinators operate independent of MORE through the various transplant centres.
In 1992, Québec Transplant, a non-profit agency independent of the healthcare system, was mandated by the Minister of Health and Social Services to manage the provincial organ donation, procurement and distribution process. It has recently turned over public awareness activities to existing provincial organizations.
Organ Procurement and Exchange of Newfoundland and Labrador (OPEN) was established in 1982. It promotes increased awareness among both the public and professionals and establishes organ procurement policies and standards. When organs are procured from any of the eight health regions, they are sent primarily to Halifax for transplantation.
Organ Procurement and Transplantation (OPT) of New Brunswick was established in 1981. The majority of organs procured in New Brunswick go to Halifax, but geography determines that some can be moved more quickly to Quebec City. If these centres do not have a suitable recipient the organs are then offered to progressively westward centres. Funding for this program is through the provincial Department of Health.
Nova Scotia Multi-Organ Transplant Program is responsible for all transplant procedures for the Maritime Provinces. It is operated out of Queen Elizabeth II Health Sciences Centre in Halifax. Its mandate is to coordinate patients for the waiting lists and to organize organ donation and transplantation for all of the Atlantic provinces. The program is responsible for promoting public awareness, providing professional education and consultation, and operating a resource centre for facilities and government. It also coordinates tissue donation for Nova Scotia and Prince Edward Island.
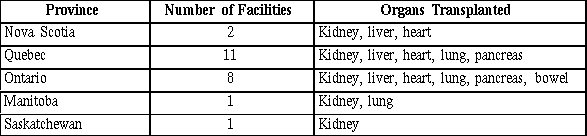
The Committee heard that a total of 28 hospitals perform organ transplants in Canada; the number of facilities carrying out tissue transplants was not provided. The majority of organ transplants performed in Canada are carried out in Ontario and Quebec. Nova Scotia is the only Atlantic province that provides transplant services. The remaining provinces have the facilities to perform at least limited transplant operations. Transplant services for individuals of the territories are provided in various provinces. As the following table suggests, virtually all types of transplant operations (with the exception of bowel) can be performed in at least one location in Ontario, Quebec, Alberta and British Columbia; Manitoba, Saskatchewan and Nova Scotia are more limited in their ability to cover all transplant procedures.
A large portion of the evidence heard by the Committee was from medical practitioners and nurses, the two primary groups of healthcare professionals involved in tissue and organ transplantation. These appeared in several different capacities: as members of transplant associations, as members of professional societies and associations, and as individuals involved at various stages of the donation/transplantation process.
The Committee heard from two professional transplant associations: the Canadian Association of Transplantation (CAT) and the Canadian Transplant Society (CTS). CAT is an association of allied health professionals such as clinical and donor coordinators, intensive care unit (ICU), critical care unit (CCU) and operating room (OR) nurses and laboratory technicians dedicated to public and professional education to improve the transplant process. As well as promoting donation and transplantation, they formulate policies and guidelines to improve the efficiency and effectiveness of procedures. The CTS is a society of the Royal College of Physicians and Surgeons of Canada whose members are transplant physicians, surgeons and scientists that meet yearly to exchange data.
There are a number of professional societies or associations within Canada whose focus is on broader aspects of healthcare but whose membership includes individuals involved in the donation/transplantation area. Some, such as the Canadian Cardiovascular Society, the Opthalmological Society, and the Fertility and Andrology Society, focus on diseases, or deficiencies, of specific organs, tissues and systems and on specific disorders that may be treated by transplantation. Other organizations, such as the Canadian Association of Critical Care Nurses and Rose des Sables, contribute directly while umbrella groups such as the Canadian Healthcare Association, the Canadian Medical Association, and the Canadian Nurses Association, represent broader interests.
Individual healthcare professionals with direct positions in this area include the transplant physicians and surgeons, intensivists, OR nurses, organ donor/trauma coordinators, clinical nurse specialists and transplant education coordinators.
Constitutionally, the provincial governments have wide powers to regulate local health matters and particularly the delivery of healthcare services; specifically, they have the authority to make laws concerning the establishment, maintenance, and management of hospitals, asylums, and charitable institutions. This gives them the primary role in healthcare activities and legal concerns relating to organ and tissue donation and transplantation.
The Committee did not hear comprehensive detail about how provincial ministries of health are involved in or support donation and transplantation programs for organs and tissues. In spite of invitations to all health ministries few provided official governmental positions. Only British Columbia's provincial Minister of Health appeared in person, while the Saskatchewan Minister of Health followed with an informative letter about provincial activities. Fragments of information on provincial government efforts were elicited from a variety of sources and are highlighted within the next part of the report where there is discussion of the various stages of the donation/transplantation process or continuum.
While the Committee did not receive a detailed picture from the health ministries of each province, it did obtain information from the Parliamentary Research Branch on the state of legislation in each province related to organs and tissues. The similarities among the provincial statutes referring to human tissues suggest that it would be a relatively easy task to implement additional regulations to ensure a nationwide legal approach. The British Columbia government indicated that it had proposed regulatory amendments to the Human Tissue Gift Act to take effect in April 1999. The regulations stipulate required referral; that is, an obligation by hospitals to inform the transplant society of the death or impending death of an individual for a determination as to whether the person is a potential donor and whether there is a registration of wishes about organ donation. The regulations will also require training for health professionals who ask for consent from families.
The realization that other countries are grappling with the same donation and transplantation issues as Canada led the Committee to invite several international witnesses to provide information on alternative models. The experience in Spain, the so-called Spanish Model operated for nine years by the Organizacion Nacional de Trasplantes (ONT), generated considerable interest. The knowledge gained by several organizations in the United States was also shared with the Committee. This included information about the State of Pennsylvania and its Delaware Valley Transplant Program; the United Network for Organ Sharing (UNOS) which organizes the national allocation system; and The Partnership for Organ Donation which focuses on hospital identification of potential donors. In addition, Donor Action (Eurotransplant, U.K.), a European-based organization working on potential donor identification, discussed its experiences.
E. FEDERAL ROLES AND RESPONSIBILITIES
The federal government's role in relation to organ and tissue donation and transplantation has been minimal. Generally, this is related to the division of constitutional powers. The federal government gains its authority in health matters through general powers, namely those pertaining to criminal law, spending, and peace, order and good government. In addition, it has specific authority for certain groups such as First Nations people on reserves, veterans, military personnel, the RCMP, and individuals within federal correctional services and its institutions.
Interpreted broadly, the federal government's key roles in the health of Canadians have been protection of their health, promotion of strategies to improve their health and support of the healthcare system. Within this context, the federal government can:
- Respond to the health needs of, and deliver healthcare services to specific groups under federal jurisdiction;
- Support the healthcare system through the Canada Health and Social Transfer;
- Monitor and administer the Canada Health Act and its five principles - accessibility, portability, comprehensiveness, public administration, and universality;
- Provide funding for research and evaluation initiatives within such bodies as the Medical Research Council and the Social Sciences and Humanities Research Council;
- Protect the health of Canadians, directly and in cooperation with other federal agencies and provincial governments, through legislation such as the Food and Drugs Act and specific regulations;
- Work in partnership with provincial and territorial governments to foster national approaches to health programs and services;
- Direct spending to specific programs and initiatives through clearly delineated and targeted strategies for improving health.
At Health Canada, the primary activity on organs and tissues has been in the area of safety standards. The Food and Drugs Act (and its Regulations) is currently the primary legislation in this area. The Health Protection Branch (HPB) administers programs to ensure safety in relation to foods, drugs, medical devices, preventable diseases, environmental pollutants, and hazardous products. With respect to the Committee's mandate, HPB has undertaken work on blood, semen and other tissues, on organs and on xenotransplantation. The Therapeutic Products Programme (TPP) is the key directorate within HPB in relation to regulatory and inspection aspects. Within TPP, several bureaux and expert groups provide significant direction on organs and tissues. The Laboratory Centre for Disease Control (LCDC), through its work in identifying, investigating, controlling and preventing human disease, can contribute to the science and the surveillance of these products.
2. Past Federal Regulatory Activity and the Proposed Standards-Based Regulatory Model
The following chronology highlights some of the key federal regulatory activities in relation to organs and tissues over the last five decades. It also describes the current status of the Canadian General Standard for Safety of Organs and Tissues for Transplantation.
1953 - The words "sera and drugs analogous thereto for parenteral use" were added to Schedule D (List of biologic drugs) of the Food and Drugs Act; this was interpreted as meaning blood products that were fractionated from plasma, not as including whole blood or blood components;
1978 - The words "human plasma collected by plasmapheresis" were added to Schedule D;
1982 - Schedule D was amended and "sera and drugs analagous thereto" was replaced by "blood derivatives";
1989 - Blood was added to Schedule D;
1993 - Royal Commission on New Reproductive Technologies reported and called for a National Regulatory Commission to ensure licensing, monitoring, standards, records storage, information dissemination for human sperm, eggs, zygotes, and fetal tissue;
1994 - Bureau of Biologics, within the Health Protection Branch (HPB), received a commissioned report from Organ Sharing Canada called Safety of Human Organ and Tissue Transplantation in Canada including recommendations for the prevention of disease transmission;
1995 - Therapeutic Products Programme (TPP), within HPB, sponsored a National Consensus Conference on "Safety of Organs and Tissues for Transplantation";
1996 - An expert advisory group, established to revise and further develop the organ and tissue standards initially drafted at the National Consensus Conference, completed the draft of the "Canadian General Standard for Safety of Organs and Tissues for Transplantation";
1996 - Regulations to ensure the safety of semen used for donor insemination were adopted under the Food and Drugs Act;
1996 - Minister of Health introduced Bill C-47, the Reproductive and Genetic Technologies Act, containing a list of prohibited practices and promised a second bill that would present the regulatory component; this legislative action was interrupted by the 1997 election call;
1997 - Commission of Inquiry on the Blood System in Canada reported and called for a national system for the collection and delivery of blood components and blood products, the creation of a national integrated database, and an active role for the federal regulator in protecting public health and safety;
1997 - Blood-borne Pathogens Division, within HPB, provided financial and scientific support to assist the Canadian Blood Agency and Canadian Medical Association in developing practice guidelines on the utilization of red cells and plasma;
1997 - TPP, within HPB, sponsored the National Forum on Xenotransplantation;
1998 - Standards for the subsets such as ocular tissue, perfusable organs, reproductive tissues, stem cells, general tissues were developed;
Towards the end of the study, the Committee received copies of the "Revised Canadian General Standard for Safety of Organs and Tissues for Transplantation" and its various subsets. The standards cover requirements for organization; facilities; consent and screening; procurement, processing, preservation, and storage; labels and inserts; quarantine; distribution; and adverse reactions/suspected infections. Health Canada emphasized that these standards are developed on the principle of consensus and once approved will be made mandatory through incorporation by reference in regulations enacted under the authority of the Food and Drugs Act.
Health Canada through the work of the Therapeutic Products Programme (TPP) developed a risk-management framework to address the safety of tissues and organs for transplantation. This standards-based regulatory framework consists of several elements:
- National standards, made mandatory under the Food and Drugs Act, to be reviewed and revised on a periodic basis;
- TPP to receive conformity assessment reports from transplant programs within one year after regulatory enactment;
- Only parties certified by TPP or accredited by standards body to assess conformity;
- TPP to require transplant program registration involving regular activity reports and adverse events reports;
- TPP inspectors to conduct periodic audits and to respond to incidents of non-compliance or serious adverse events.
3. Federal Involvement in National Endeavours
During the 1990s, the federal government, specifically Health Canada, was involved in several federal, provincial and territorial incremental advisory endeavours specific to organs and tissues. In 1995, in response to the earlier report by Organ Sharing Canada called Safety of Human Organ and Tissue Transplantation in Canada, the provinces' deputy ministers of health asked the Federal/Provincial/Territorial Advisory Committee on Health Services (ACHS) to review organ transplantation. It did so and in September 1996, it produced a document called Organ and Tissue Donation and Distribution in Canada which identified a 13-point national strategy for improving organ/tissue donation and distribution. In 1997 the ACHS produced an implementation plan with a description of each of the 13 elements, a timeframe, a cost and identification of specific organizations to take the lead and specific organizations to provide support.
In May 1997, the National Advisory Committee on Organ and Tissue Donation and Distribution (subsequently renamed the National Coordinating Committee, NCC) was appointed and asked to further research the implementation requirements for each of the 13 points. The NCC is composed of five governmental members (western region, Quebec, Ontario, eastern region, Health Canada) and five non-governmental members (Canadian Transplant Society, Canadian Association of Transplantation, Canadian College of Health Services Accreditation, Kidney Foundation, Canadian Institute of Health Information). The NCC reports semi-annually to the ACHS. It is co-chaired by a transplant physician and a provincial government director of acute care programs. It was allocated $500,000 for a 3-year period; $150,000 is used to maintain secretariat and operating support while the remaining $350,000 will support specific contracts to analyze options for implementation.
One group involved in the 13-point national plan is the Coordinating Committee on Reciprocal Billing (CCRB). Created in 1991 to resolve administrative complexities arising from interprovincial arrangements, it recently addressed the costs of transplantation, particularly that of organs where individual recipients often must cross provincial and territorial borders. While the membership of the committee is limited to four provinces with Health Canada providing the secretariat support, all provinces and territories are involved and any concerns can be referred to F/P/T Advisory Committee on Health Services (ACHS).
Beyond this type of time-limited or authority-limited coordinating committee described above, various permanent national bodies, operational in the 1990s, reflect partnerships forged in federal, provincial and territorial forums with relevance to organs and tissues. Initiatives endorsed by the F/P/T ministers of health have resulted in greater co-operation and coordination in matters related to organs and tissues. The following section outlines some of the key organizations resulting from these efforts: the Canadian Coordinating Office for Health Technology Assessment (CCOHTA), the Canadian Institute for Health Information (CIHI), and the Canadian Blood Services (CBS).
In 1989, the Canadian Coordinating Office for Health Technology Assessment was established by the F/P/T ministers of health to facilitate information exchange and to coordinate assessment of healthcare technologies. Its mandate was expanded in 1993 to include pharmaceutical assessments. CCOHTA is a non-profit corporation funded by the federal and provincial governments with a board of directors appointed by the deputy ministers of health. Its 1999-2000 funding of $1.78 million is 30% federal and 70% provincial on a per capita basis. In 1997, it engaged in a project to examine the criteria for selection of adult recipients for heart, kidney, and liver transplantation.
In 1993, based on the agreement of the F/P/T ministers of health, the Canadian Institute for Health Information was created from two not-for-profit non-government organizations (Management Information Systems Group and Hospital Medical Records Institute) and health information groups from Health Canada and Statistics Canada. Its initial funding was split between Health Canada and provincial/territorial ministries of health. Now, its funding totals about $13 million, with approximately 65% from federal, provincial and territorial governments, 25% from healthcare facilities, 14% from user fees, and 1% from other revenues. As an independent, not-for-profit federally incorporated organization, CIHI is mandated to lead the development and maintenance of a comprehensive health information system. Its 15-member board of directors consists of a president, two members from each of five regions (Atlantic, Quebec, Ontario, NWT and Prairies, Yukon and B.C.), two from the federal government (Health Canada and Statistics Canada) and two from non-governmental areas. Early in its term, it assumed responsibility for the Canadian Organ Replacement Register (CORR), a national database that records, analyzes and reports the levels of activity and outcomes of vital organ transplantation.
In 1998, the federal and provincial health ministers established the Canadian Blood Services whose 1999-2000 budget of $536 million is exclusively provincial. While its initial funding was from the federal government, continuing operational funding will be from the provinces with the federal government contributing $5 million to research beginning in 2001. The CBS mandate is to be responsible for a national blood supply system that assures access to a safe, secure, affordable supply of blood, blood products and alternatives. It recruits and manages blood donors; collects, tests, processes, stores and distributes blood; develops and implements quality control standards; establishes public and professional education programs; purchases and distributes commercial blood products; and conducts surveillance. Héma-Québec, also established in 1998, is subject to the same federal regulatory requirements under the Food and Drugs Act. The CBS board of directors includes representatives of regional, consumer, medical, scientific and public health interests.
* Individual terms, bolded in this fashion, can be found in the Glossary.