HESA Committee Report
If you have any questions or comments regarding the accessibility of this publication, please contact us at accessible@parl.gc.ca.
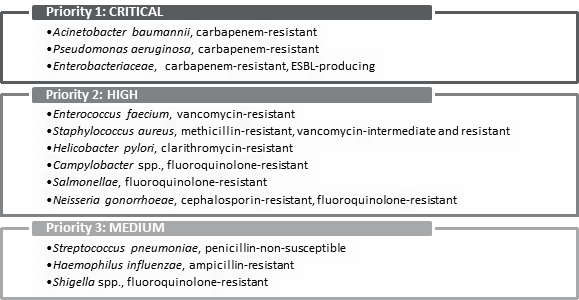
INTRODUCTIONOn 7 March 2016, the House of Commons Standing Committee on Health (the committee) agreed to undertake a study on the status of antimicrobial resistance (AMR) in Canada.[1] The committee held six meetings with witnesses (two in June 2017, and four in November 2017), and received 17 briefs relating to this study. Over the course of its meetings on AMR, the committee heard from government officials, academics, international organizations and stakeholders in the fields of both human and animal health. This report first explains AMR and its causes, and then summarizes the scope of the AMR issue, both in Canada and globally. Next, it describes how AMR is being addressed in the international context, and sets out Canada’s approach to addressing AMR. Finally, the report summarizes the challenges that Canada faces in addressing AMR (as identified by witnesses), and makes recommendations to address those challenges. AMR is “one of the most serious global health threats facing the world today,” with significant impacts both domestically and internationally.[2] These impacts extend beyond health, to agriculture, trade, and the environment, making it a complex problem to address. WHAT IS ANTIMICROBIAL RESISTANCE?An antimicrobial is a natural, semisynthetic or synthetic substance that is used to inhibit the growth of microorganisms, such as bacteria, viruses, fungi and parasites, which can cause infections.[3] Antimicrobial drugs include antibiotics, antivirals, antifungals and antiparasitics. The World Health Organization (WHO) explains that “[a]ntimicrobial resistance [AMR] occurs when microorganisms such as bacteria, viruses, fungi and parasites change in ways that render the medications used to cure the infections they cause ineffective.”[4] Once entrenched within a population or community, infections involving these microbes become increasingly difficult to treat.[5] According to Dr. Andrew Morris, Chair, Antimicrobial Stewardship and Resistance Committee, Association of Medical Microbiology and Infectious Disease Canada, AMR not only affects the management of common infections such as pneumonia and urinary tract infections, but it can also affect patients undergoing routine surgeries who are at risk of being infected with antimicrobial-resistant pathogens.[6] Dr. Neil Rau, Infectious Diseases Specialist and Medical Microbiologist, Halton Health Care, further explained that the impact of AMR on human health outcomes is dependent upon the interaction of three main factors referred to as “The Epidemiologic Triangle”: the health of the individual, the environment that the individual lives in, and the microbe or agent itself (see Figure 1).[7] For example, Dr. Rau explained that an individual hospitalized because of a prior chronic condition will face greater risks from an antimicrobial resistant pathogen because his or her immune system is compromised by their underlying condition and the impact of their prolonged period of stay in a hospital, whereas an otherwise healthy individual who ends up in the emergency room with a broken arm may face less risk when exposed to the same agent.[8] Similarly, at a population health level, rates of drug-resistant infections are affected by the aging population and changing health technologies that allow for a greater number of surgeries and transplants, which means more patients are at risk of being exposed to anti-microbial resistant infections.[9] Figure 1. “The Epidemiologic Triangle”: Host and Environmental Factors Play Important Roles in Antimicrobial Disease Outcomes
Source: adapted from: Dr. Neil V. Rau, Infections Disease Specialist and Medical Microbiologist, Halton Health Care, “Antimicrobial Resistance,” submitted to HESA on 7 November 2017. WHAT CAUSES ANTIMICROBIAL RESISTANCE?While AMR can occur naturally or from the use of antimicrobials, the committee heard from Dr. Howard Njoo, Deputy Chief Public Health Officer for the Public Health Agency of Canada (PHAC), that the inappropriate use of antimicrobials in health care, sanitation, animal health and food production accelerates its emergence and spread.[10] According to Dr. Wendy Levinson, Chair, Choosing Wisely Canada, the inappropriate use of antimicrobials in health care settings arises from various factors, including clinicians facing pressures to prescribe medications to patients, as well as having limited time available to provide them with other treatment options.[11] In addition, clinicians lack health information technology systems to support or provide feedback on prescribing practices. Dr. Rau also explained that family physicians have limited access to diagnostic tests that enable them to decipher whether individuals have a viral or bacterial infection.[12] Within the animal health and food production sectors, antimicrobials have been used widely for production purposes to promote growth, as well as to treat and prevent diseases in animals. Dr. Scott McEwen, Professor, Ontario Veterinary College, University of Guelph, explained that overuse of antimicrobials contributes to the emergence of antimicrobial resistant pathogens in both food-producing and companion animals.[13] However, food-producing animals pose the greatest risk to the spread of AMR because of the relatively large amount of antimicrobials that are used in that sector. In addition, antimicrobial-resistant pathogens among food production animals pose the greatest risk to human health as they can be transferred to humans through the food supply chain, causing treatment-resistant food-borne illnesses among humans.[14] At the same time, Dr. Rau noted that “[t]here is truth to the link between farm use of antibiotics for animal husbandry purposes and drug resistance, but I don’t think it applies so much in Canada.”[15] WHAT IS THE SCOPE OF THE PROBLEM?The committee heard from Dr. Njoo that AMR is “one of the most serious global health threats facing the world today,” with significant impacts both domestically and internationally.[16] These impacts extend beyond health, to agriculture, trade, and the environment, making AMR a complex problem to address. An overview of the extent to which AMR poses a threat both domestically and internationally is outlined in the sections below. A. Global Impacts of Antimicrobial ResistanceThe committee heard that tuberculosis (TB) poses the greatest threat to human health and development, as drug-resistant TB is the most common and lethal airborne antimicrobial-resistant disease worldwide today, responsible for 250,000 deaths each year.[17] Furthermore, it accounts for one-third of deaths caused by AMR globally.[18] Consequently, the WHO declared TB a national and global health emergency in 1993. In addition, the committee heard that in 2017, the WHO released a three-tiered list of priority pathogens that are resistant to treatment that pose the greatest risk to human health and for which there are limited treatment options for treatment available (see Figure 2).[19] The three most critical resistant pathogens on tier one of the WHO list are found primarily in hospital environments and pose the greatest risk to individuals in intensive care units or burn units; individuals who are receiving transplants; individuals with Cystic Fibrosis and oncology patients.[20] Though these antimicrobial-resistant pathogens are most common in southern Europe, the Middle East and Asia, they can cross borders through international travel and migration, transforming AMR into a global health threat. Other high-priority pathogens listed in tier two include Salmonella species and Campylobacter species, which cause food-borne illnesses, and Neisseria gonorrhoeae, which causes gonorrhea, a community-acquired sexually transmitted infection.[21] Staphylococcus aureaus infections are also considered high priority and may be transferred through community or hospital settings without any prior exposure to antibiotics.[22] Figure 2. World Health Organization Priority Pathogens List for R&D of New Antibiotics
Source: World Health Organization, “WHO publishes list of bacteria for which new antibiotics are urgently needed.” In terms of estimating the global impact of AMR, Dr. Njoo from PHAC referred to the United Kingdom’s 2016 Review on Antimicrobial Resistance,[23] which “estimated that annual worldwide human deaths attributable to AMR could reach 10 million by 2050. This figure would overtake the number of deaths resulting from diabetes and cancer combined.”[24] In terms of the economic impact on the global economy, Dr. Timothy G. Evans, Senior Director, Health, Nutrition and Population Global Practice, World Bank Group, explained to the committee that costing simulations had found that: in the optimistic case of low impact, AMR by 2050 would amount to a reduction of 1.1% of global GDP. By 2030 this would shave about $1 trillion off global GDP annually. In the high-impact scenario, the reduction of global GDP in 2050 would be 3.8% with an annual shortfall of $3.4 trillion in global GDP as of 2030.[25] B. Antimicrobial Resistance in Middle- and Low-Income CountriesWitnesses further explained that the wide ranging impacts of antimicrobial resistance would be most acutely felt in middle- and low-income countries. RESULTS Canada stated in its brief to the committee, “[t]hough antimicrobial resistance (AMR) is developing across borders with far-reaching global impact, the diseases under the threat of developing drug resistance often disproportionately affect poorer and marginalized communities – including HIV, malaria, and tuberculosis.”[26] Dr. Rau further explained how environmental conditions in low- and middle-income countries affect the spread of AMR: TB is a huge problem, especially in developing countries. Again I speak to the epidemiologic triangle. There's a whole factor about the environment: people live in crowded conditions, so there's more spread; they don't have access to care; they don't get proper rounds of first-line therapy, so they have resistance generated because they're being improperly treated. That's an environment factor that's driving resistance. Is this a problem? Of course it is. There need to be new drugs for TB. A few are in the offing, but this has been an ignored disease.[27] Finally, Dr. Evans from the World Bank explained that of the 28 million people that could be impoverished by AMR by 2050, the majority would live in low-income countries.[28] According to Dr. Evans, AMR may exacerbate poverty in low- income countries through its reduction in international trade relating to livestock and livestock products, which serve as a significant portion of income for individuals in those countries. In addition, AMR may increase the costs of health care in low-income countries by as much as 25% due to caring for patients with more complicated conditions.[29] He therefore concluded that “AMR is not just a health care issue; it is a development issue, which if unaddressed threatens to derail economies and the achievement of our most fundamental development goal at the World Bank, which is to eliminate extreme poverty.”[30] C. Antimicrobial Resistance in CanadaIn terms of the scope of the problem in Canada, more than 18,000 hospitalized patients acquire infections that are resistant to antimicrobials every year, according to PHAC.[31] The committee heard that many of these patients, whose health is already compromised, may suffer and die unnecessarily as a result of these infections.[32] However, because of inadequate surveillance data, Dr. Morris explained that mortality rates arising from AMR in Canada remain unknown.[33] In terms of antimicrobial-resistant pathogens posing a particular threat in Canada, Dr. Marc Ouellette, Scientific Director, Infection and Immunity, Canadian Institutes of Health Research (CIHR), explained that methicillin-resistant Staphylococcus aureus (MRSA) remains a significant threat, as methicillin is the main line of treatment for infections caused by this pathogen.[34] He further explained that though there is now an effective vaccine available for subgroups of Streptococcus pneumoniae, these bacteria continue to evolve and develop resistance to antimicrobials. Dr. Morris told the committee that up to half of the bacteria causing infections among cancer and surgery patients are already resistant to first-line antibiotics in the United States. However, he noted that, “I would like to quote Canadian data, but we don’t really have reliable ones. It is likely comparable.”[35] Finally, in their written submission to the committee, Doctors Without Borders and the International Union Against Tuberculosis and Lung Disease, indicated that TB affects over 1,600 people in Canada, with a mortality rate of 8%.[36] Though these rates have remained stable for the past decade, the brief explains that TB disproportionally affects Indigenous communities in Canada, particularly Inuit communities, where incidence rates reach 166.2 per 100,000 compared to the national rate of 4.4 per 100,000. However, the committee heard that cases of AMR TB remain infrequent, “In Canada, a case of XDR (extensively drug resistant TB) would be extremely rare. I think there has been one or two cases in the last five or six years. Multidrug-resistant TB is a bit more common. There’s a handful of cases every year.”[37] HOW IS ANTIMICROBIAL RESISTANCE BEING ADDRESSED GLOBALLY?The committee heard that international organizations, as well as individual countries, are addressing AMR in various ways. Some countries have adopted innovative approaches, such as diagnostic strategies and prescribing practices, to promote antimicrobial stewardship. A. Frameworks to Address Antimicrobial Resistance GloballyOn 21 September 2016, United Nations (UN) member states agreed by acclamation to each develop and adopt a national action plan to combat AMR. In addition, the national action plans would have to comply with the parameters outlined by the WHO in its Global Action Plan on Antimicrobial Resistance (2015). The committee heard that Canada is contributing $9 million to support the implementation of the WHO’s global action plan on AMR and is also taking the lead in supporting the implementation of WHO’s action plan through the Global Health Security Agenda,[38] a partnership of nearly 50 nations, international organizations, and non-governmental organizations that are working to build national and international capacity to address threats from infectious diseases.[39] The World Organization for Animal Health (OIE) “is the intergovernmental organization responsible for improving animal health worldwide.”[40] The OIE has a tripartite alliance with the WHO and the Food and Agriculture Organization of the United Nations (FAO), and one of the alliance’s priorities is AMR.[41] As Dr. Landals explained, “Both the OIE and the World Health Organization have stressed the importance of raising awareness of health risks posed by antibiotic resistance and promoting good practice in how we use these drugs to limit the emergence of antimicrobial resistance.”[42] At the same time, Dr. Mary-Jane Ireland, Director General of the Veterinary Drugs Directorate at Health Canada, noted the following: I would consider that preventing disease in a particular herd for which it's known there's a disease pattern, as well as treating a disease, is important. Preventing disease, and the snowball effect of many more animals becoming much more sick, and having to use more antimicrobials, and maybe second line and third line treatments, is a reasonable practice.[43] The WHO guidelines on the use of medically important antimicrobials in food-producing animals were recently released, and “identify several important restrictions on the use of these antimicrobials in animals, restrictions that should be implemented in all countries.”[44] B. New Diagnostic StrategiesAMR affects different regions of the world and not all are affected in the same way. As Dr. Ouellette explained, “there are regions of the world where there are no more antibiotics that are capable of treating some very bad bugs.”[45] This situation has led some countries to develop new diagnostic strategies. For example, Kuwait, which has higher rates of drug resistance than Canada, uses precision testing for resistance markers.[46] Biomarkers can be used to determine whether an illness is bacterial in origin. Once determined, an appropriate treatment action is then prescribed, which can reduce unnecessary antibiotic use. These strategies were explained by Dr. Rau: There is a point-of-care version, which is used in Nordic countries, and also another test, called CRP or C-reactive protein. The other one, which is used in hospitals a lot—especially in Europe and the Middle East, but, increasingly, some places in North America are also looking at this—is the procalcitonin test,[47] especially in intensive care units, as a way of helping antimicrobial stewardship teams decide when to stop antibiotics.[48] In Nordic countries, many family physicians have access to CRP and procalcitonin testing.[49] C. Antibiotic Use GuidelinesTreatment protocols or antibiotic use guidelines are one way in which other countries are addressing AMR. For example, with respect to ear infections in children in the Netherlands and Nordic countries, [children] are observed for 48 hours before giving them antibiotics. In Canada, however, the reflex is to give them antibiotics because an ear infection can sometimes cause meningitis, and the infection, if undiagnosed, can lead to many long-term complications. To avoid a single possible case of harmful complications, a hundred people are treated with antibiotics.[50] D. Financial Investment in Antimicrobial Resistance-Related Research and DevelopmentWith respect to funding for AMR initiatives, the committee was told that Canada’s investment in AMR is low compared to some countries: there are many countries, including the U.S., the United Kingdom, Australia, Germany, the Netherlands, Scandinavian countries, etc., that are putting in substantially more relative investment than Canada is, and have already started significant work and put in significant investments. In the U.S. alone, there's a presidential advisory committee on AMR with important national leaders getting together and advising on where investments should go. We don't have the investments to advise our leaders on where it should go.[51] A public-private partnership in the U.S. known as the CARB-X (combatting antibiotic-resistant bacteria biopharmaceutical accelerator) Global Partnership funds innovation, “encouraging development of new medicines and rapid diagnostics.”[52] In Europe, the Innovative Medicines Initiative is “stimulat[ing] antibiotic discovery…to the tune of 700 million euros.”[53] E. Antibiotic Use in Food AnimalsDr. McEwen explained to the committee how antibiotic use in food animals is being addressed in Europe: Some European countries, such as France, the Netherlands, and the United Kingdom, have made up to 50% reductions in consumption of these antimicrobials in animals by setting national targets and, in the case of the Netherlands and Denmark, by measuring antimicrobial consumption at the farm and veterinary clinic levels and implementing strategies to encourage veterinarians and farmers to do their part to meet these targets.[54] HOW IS ANTIMICROBIAL RESISTANCE BEING ADDRESSED IN CANADA?The committee heard that organizations in both the public and private sector have been working to address AMR in Canada. Dr. Njoo from PHAC explained that many of these activities are being coordinated through two main strategic plans: Antimicrobial Resistance and Use in Canada: A Federal Framework for Action[55] and Tackling Antimicrobial Resistance and Antimicrobial Use: A Pan-Canadian Framework for Action.[56] An overview of these strategies and activities is provided in the sections below. A. Antimicrobial Resistance and Use in Canada: A Federal Framework for ActionAccording to Dr. Njoo, the federal framework for AMR and antimicrobial use (AMU) was developed in 2014.[57] Its associated action plan, which followed in March 2015, focuses on actions to be taken by federal departments and agencies to address AMR in three main areas: surveillance, stewardship and innovation. With respect to surveillance, the committee heard that the federal government has focused its efforts on the development of The Canadian Antimicrobial Resistance Surveillance System (CARSS), which synthesizes data from PHAC surveillance systems to provide an integrated picture of both in Canada. To date, CARSS has released two reports that provided a better understanding of AMR in Canada. Dr. Njoo explained that PHAC is now examining ways to expand CARSS to include data on AMR and AMU from community health settings. The committee also heard that the Canadian Food Inspection Agency (CFIA) is contributing to CARSS through the Canadian Integrated Program for Anti-Microbial Resistance Surveillance (CIPARS), which monitors AMR in the agri-food and agriculture sectors.[58] Under the federal framework, stewardship refers to “conserving the effectiveness of existing treatments through infection prevention and control guidelines, education and awareness, regulations, and oversight.”[59] In support of this second pillar, Dr. Njoo explained that PHAC continues to engage activities to educate and raise awareness of AMR among Canadian families to promote behaviors that prevent the spread of infections, such as handwashing.[60] Education and awareness activities are also being used to help explain to Canadians why antibiotics are not always necessary, particularly in the case of viral infections. Tools and supports are also being given to prescribers to help them talk to patients about the appropriate use of antibiotics. In terms of regulatory changes to support antimicrobial stewardship, the committee heard that Health Canada introduced regulatory changes in May 2017 to reduce the use and promote oversight of the use of antimicrobials in the agri-food and agriculture sectors, including:
In addition, Health Canada is proposing that all medically important antimicrobials require a prescription for their use in animals.[62] In line with international best practices, the department is also removing growth promotion claims from the labels of antimicrobials used in animals. Finally, with respect to human drugs, the department is introducing statements on drug labels that encourage prudent prescribing by reminding prescribers to check for a patient’s susceptibility to bacterial infection prior to prescribing. The committee heard that CIHR is responsible for taking the lead on the innovation pillar of the federal framework. Dr. Marc Ouellette, Scientific Director, Infection and Immunity, CIHR explained that the organization had invested $96 million in AMR research between 2011 and 2016 that had supported the development of new antibiotics and novel therapies, new diagnostics, antimicrobial stewardship strategies, surveillance and infection prevention and control measures.[63] New investments of $1.8 million a year announced in Budget 2015 are being used to support research to address the health changes posed by antimicrobial-resistant infections. In particular, the committee heard that this funding is being provided to research teams that are developing tests to diagnose AMR rapidly at the point of care. [64] Dr. Ouellette explained that Canada is also contributing $9.7 million to support global research aimed at addressing AMR through the Joint Programming Initiative on Antimicrobial Resistance (JPIAMR). B. Tackling Antimicrobial Resistance and Antimicrobial Use: A Pan-Canadian Framework for ActionDr. Howard Njoo, Deputy Chief Public Health Officer, PHAC explained that federal action alone would not be enough to address AMR in Canada.[65] Consequently, there is a need for a broader strategy that coordinates Canada’s response to AMR across different levels of government, professional organizations, non-governmental organizations, academic institutions, industry and experts in both human and animal sectors. As a result of this need, the committee heard that federal, provincial and territorial governments, the medical and veterinary communities, industry and academia have come together to develop Tackling Antimicrobial Resistance and Antimicrobial Use: A Pan-Canadian Framework for Action,[66] which was released in September 2017. This pan-Canadian framework takes a “one health approach” to AMR that involves collaboration between human health, animal health and agriculture sectors.[67] It has four main areas of focus: surveillance, infection prevention and control, stewardship and research and innovation. The committee heard that the framework is a high level strategic document that will be followed by the development of a concrete action plan with timelines and measurable outcomes.[68] CURRENT CHALLENGES IS ADDRESSING ANTIMICROBIAL RESISTANCE IN CANADAWhile many witnesses saw both the federal and pan-Canadian frameworks as important first steps, they explained that more concrete action needs to be undertaken to address AMR in Canada, particularly in relation to surveillance, stewardship, and research and innovation. Witnesses also identified the need for greater federal leadership on the issue. A. SurveillanceMany witnesses pointed to the need for improved AMR surveillance and data. Dr. Morris from the Association of Medical Microbiology and Infectious Disease Canada stated that “[w]e don't really have a very good understanding of our current state in terms of antimicrobial resistance or in terms of antimicrobial use. I think the first efforts would have to be foundational efforts toward ensuring that we have good data.”[69] He stated that CARSS “relies on data of very poor quality…We have no current understanding of antimicrobial use in Canada and in most provinces. It’s the same problem that we’ve had with opioids. If you can’t properly, reliably, and validly identify the problem, it’s very difficult to act on it.”[70] Dr. Susanne Rhodenizer Rose stated that Canada lags behind other countries when it comes to “tracking incidents of resistant bacteria and analyzing the success of our collective interventions.”[71] While Dr. Rose stated that the establishment of CARSS is an “important first step in defining priority resistant organisms to conduct surveillance on…this is but one piece, and the potential data from this system can complement the data from a national repository for health care associated infections.”[72] For example, she explained that there are other surveillance systems in place in Canada that provide reliable and consistent data from health care settings, such as the Canadian nosocomial infection surveillance program and the Canadian Network for Public Health Intelligence, but they need to be scaled up and integrated into a national system.[73] Dr. Rau from Halton Healthcare emphasized the need for better laboratory surveillance: I think what we're doing right now is looking backward and saying that in the last two years we've had a problem. We don't have real-time surveillance to know where resistance rates are increasing. We have hospital labs that work in a silo separate from reference labs for each province. We have each province working in a silo separate from other provinces. We need a very good, integrated lab information system for tracking the rates of resistance to drugs in bloodstream infections, urinary tract infections, ICU patients. We need to have that data at our fingertips so that we know what our rates are. Once we know what our rates are, then we know how much need we have for unusual antibiotics that are hard to come by, except through a special access program.[74] Dr. Njoo from PHAC acknowledged the existing surveillance gaps: Working with our partners, we have a clear understanding of the gaps in information that need to be addressed. Key among these is the lack of human health data in the community setting. We have taken steps to assess the feasibility of collecting more and better information from community settings so that we can talk about the complete human health AMR and AMU situation.[75] B. StewardshipAccording to the Canadian Nurses Association, antimicrobial stewardship can be defined as the “practice of minimizing the emergence of antimicrobial resistance by using antibiotics only when necessary, and if needed, by selecting the appropriate antibiotic at the right dose, frequency and duration to optimize outcomes while minimizing adverse effects.”[76] While the committee heard that many hospitals and long-term care facilities have established (or are establishing) stewardship programs, Sheila Dattani from the Canadian Pharmacists Association noted that “80% of antibiotics are prescribed in the community, where few formal antimicrobial stewardship programs currently exist.”[77] Dr. Morris noted that “[c]hanging how we use antibiotics is a complicated task… It requires behavioural change techniques, psychology, infrastructure, and making it easier to do the right thing. All of those things are difficult.”[78] Witnesses emphasized the importance of education for both physicians and for patients with respect to prescribing, and using, antimicrobials appropriately. Dr. Morris recognized the importance of antibiotic use guidelines, but noted the challenge of doing so in Canada: If we're talking about guidelines, I think many experts in the field—and I consider myself one of them—recognize that in order to discuss appropriateness of antibiotic use, you need to have a benchmark. The benchmark in most countries that have done this has been to develop guidelines. We have no national guidelines on how to use antibiotics. To do that would require Herculean effort and considerable time and cost. It's almost certainly necessary, but I don't see it happening in the next five to six years.[79] The committee also heard that it is necessary to engage clinicians at the grassroots level to take a leadership role in improving their prescribing practices through initiatives such as Choosing Wisely, which is a campaign that challenges medical specialties to identify five tests and treatments that are clinically unnecessary or potentially harmful and develop recommendations on how to avoid these practices.[80] Through Choosing Wisely, clinicians have developed 20 recommendations specifically addressing the inappropriate use of antibiotics.[81] With respect to antimicrobial stewardship and food animals, Steve Leech from Chicken Farmers of Canada explained to the committee that “[w]hen we look at putting in place reduction strategies, we look at using different tools, feed alternatives, and these types of things. Unfortunately, Canadian farmers don't have access to the products that are available in Europe or even in the United States. An example of this would be probiotics.”[82] C. Research and InnovationWith respect to research and innovation, witnesses highlighted some of the research that is underway in Canada, noting that Canada has “cultivated some of the best experts in the field of AMR.”[83] Witnesses also emphasized the need for research and innovation funding. Dr. Morris stated that antimicrobial stewardship and resistance research funding is less than $10 million per year,[84] and that “very few trainees want to get into the field of AMR because there are no dollars in it.”[85] Dr. Gerard Wright, Professor, Department of Biochemistry and Biomedical Sciences, McMaster University pointed to investments made in the U.S. and the EU, noting that “Canada is nowhere to be found on this scale yet.”[86] In particular, Dr. Wright emphasized the need for Canada to target its research funding to support researchers both in Canada and abroad in transforming their discoveries in the laboratories into treatments, as this funding is not coming from pharmaceutical companies.[87] The committee heard that this could be done through public-private partnerships and increased support for initiatives such as the Canadian anti-microbial innovation network, which brings together researchers and academics from small and medium-sized enterprises to help develop new vaccines and alternatives to antibiotics.[88] D. Federal LeadershipMoving beyond the four pillars of the Pan-Canadian Framework, witnesses overwhelmingly called for federal leadership and coordination on AMR.[89] Dr. Morris stated that “we don’t really have a national voice on antimicrobial resistance and stewardship.”[90] As Dr. Michael Routledge from the Royal College of Physicians and Surgeons explained: Canada has, and has had in the past, many examples of local pockets of excellent work on antimicrobial resistance, the Do Bugs Need Drugs? program in B.C. and Alberta being one example. What has primarily been lacking is a robust structure that can coordinate, disseminate, and support these leading practices across all health care organizations and professionals in Canada. The creation over the past few years of the 2014 federal framework and the current FPT steering process, combined with the efforts of organizations like HealthCareCAN and the NCCID, have positioned Canada well to take the necessary next steps.[91] MOVING FORWARD TO COMBAT ANTIMICROBIAL RESISTANCE: COMMITTEE RECOMMENDATIONSMany witnesses emphasized Canada’s successes with respect to AMR, but also stressed the importance of not losing momentum on the issue,[92] and improving collaboration and coordination, both at the national level and globally. Witnesses also emphasized that Canada could be a leader in combatting AMR,[93] particularly given that it is hosting the G7 in 2018, and is chairing “the global health security agenda’s AMR action package.”[94] The committee agrees that Canada has made significant efforts to tackle AMR. At the same time, the committee stresses that more needs to be done, particularly with respect to federal leadership and coordination and investment in research and innovation. The committee also acknowledges the need for improved education for physicians and patients in relation to appropriate prescribing practices for antimicrobials, but recognizes the role of the provinces with respect to that particular issue. The committee therefore recommends: The Forthcoming Pan-Canadian Action Plan for the Tackling Antimicrobial Resistance and Antimicrobial Use: A Pan-Canadian Framework for Action Recommendation 1 That the Public Health Agency of Canada accelerate the development of the Pan-Canadian Action Plan for the Tackling Antimicrobial Resistance and Antimicrobial Use: A Pan-Canadian Framework for Action. The Pan-Canadian Action Plan should include measurable goals and targets, as well as clear timelines for implementation. Federal Leadership Recommendation 2 That, as part of the forthcoming Pan-Canadian Action Plan for the Tackling Antimicrobial Resistance and Antimicrobial Use: A Pan-Canadian Framework for Action, the Public Health Agency of Canada appoint a federal advisor to be the national champion for combatting antimicrobial resistance across Canada. Recommendation 3 That, as part of the forthcoming Pan-Canadian Action Plan for the Tackling Antimicrobial Resistance and Antimicrobial Use: A Pan-Canadian Framework for Action, the Public Health Agency of Canada emphasize global leadership, with targeted investments for research and development of drugs and other treatments for drug-resistant tuberculosis. Antimicrobial Stewardship Recommendation 4 That the Public Health Agency of Canada ensure that the forthcoming Pan-Canadian Action Plan for the Tackling Antimicrobial Resistance and Antimicrobial Use: A Pan-Canadian Framework for Action focus on identifying ways to scale up current best practices in antimicrobial resistance stewardship across Canada. Recommendation 5 That, as part of the forthcoming Pan-Canadian Action Plan for the Tackling Antimicrobial Resistance and Antimicrobial Use: A Pan-Canadian Framework for Action, the Public Health Agency of Canada work with the provinces and territories and their health professional regulatory bodies to develop educational materials for physicians and nurse practitioners, and for the public, in relation to the responsible use of antimicrobials. Recommendation 6 That, as part of the forthcoming Pan-Canadian Action Plan for the Tackling Antimicrobial Resistance and Antimicrobial Use: A Pan-Canadian Framework for Action, the Public Health Agency of Canada work with the provinces and territories and their health professional regulatory bodies to develop voluntary national prescribing guidelines. Recommendation 7 That, as part of the forthcoming Pan-Canadian Action Plan for the Tackling Antimicrobial Resistance and Antimicrobial Use: A Pan-Canadian Framework for Action, Health Canada consider improving access to alternative therapies for food animals, such as probiotics, to reduce the use of antimicrobials in food animals. Surveillance Recommendation 8 That, as part of the forthcoming Pan-Canadian Action Plan for the Tackling Antimicrobial Resistance and Antimicrobial Use: A Pan-Canadian Framework for Action , the Public Health Agency of Canada expand the role of The Canadian Antimicrobial Resistance Surveillance System to integrate and scale up existing data systems. Funding Recommendation 9 That, as part of the forthcoming Pan-Canadian Action Plan for the Tackling Antimicrobial Resistance and Antimicrobial Use: A Pan-Canadian Framework for Action, the Government of Canada provide stable and adequate funding to support research and innovation in the area of antimicrobial resistance, including support for small and medium-sized enterprises to bridge the “valley of death”; the promotion of public-private partnerships; and the promotion of or funding for behavioural research to address antimicrobial overprescribing practices from both a patient and physician/nurse practitioner perspective. Recommendation 10 That, as part of the forthcoming Pan-Canadian Action Plan for the Tackling Antimicrobial Resistance and Antimicrobial Use: A Pan-Canadian Framework for Action, the Government of Canada explore the possibility of funding a network of centres of excellence on antimicrobial resistance. [1] House of Commons, Standing Committee on Health (HESA), Minutes of Proceedings, 1st Session, 42nd Parliament, 7 March 2016. [2] HESA, Evidence, 13 June 2017, 1100 (Dr. Howard Njoo, Deputy Chief Public Health Officer, Acting Assistant Deputy Minister, Infectious Disease Prevention and Control Branch, Public Health Agency of Canada). [3] Public Health Agency of Canada (PHAC), Canadian Antimicrobial Resistance Surveillance System – Report 2016, September 2016. [4] World Health Organization, “What is antimicrobial resistance?,” July 2017. [5] Ibid. [6] Ibid. [7] HESA, Evidence, 7 November 2017, 1600 (Dr. Neil Rau, Infectious Diseases Specialist and Medical Microbiologist, Halton Healthcare). [8] Ibid. [9] Ibid. [13] HESA, Evidence, 9 November 2017, 1605 (Dr. Scott McEwen, Professor, Ontario Veterinary College, University of Guelph, As an Individual). [14] Ibid. [17] HESA, Evidence, 21 November 2017, 1625 (Mr. Willo Brock, Senior Vice-President, External Affairs, TB Alliance). [18] Ibid. [19] Dr. Neil V. Rau, Infections Disease Specialist and Medical Microbiologist, Halton Health Care, “Antimicrobial Resistance,” submitted to HESA on 7 November 2017. [20] Ibid. [21] Ibid. [22] Ibid. [23] Review on Antimicrobial Resistance (Chair, Jim O’Neill), Tackling Drug-Resistant Infections Globally: Final Report and Recommendations, May 2016. [25] HESA, Evidence, 21 November 2017, 1550 (Dr. Timothy G. Evans, Senior Director, Health, Nutrition and Population Global Practice, World Bank Group). [26] RESULTS Canada, Brief, Tuberculosis: The Heart of the Global Antimicrobial Resistance Crisis, 7 November 2017. [29] Ibid. [30] Ibid. [31] Government of Canada, “Antimicrobial Resistance and Use in Canada: A Federal Framework for Action,” October 2014. [32] HESA, Evidence, 7 November 2017, 1610 (Ms. Sandi Kossey, Senior Director, Strategic Partnerships and Priorities, Canadian Patient Safety Institute). [33] HESA, Evidence, 15 July 2017, 1225 (Dr. Andrew Morris, Chair, Association of Medical Microbiology and Infectious Disease Canada). [34] HESA, Evidence, 13 June 2017, 1158 (Dr. Marc Ouellette, Scientific Director, Infection and Immunity, Canadian Institutes of Health Research (CIHR)). [36] Doctors Without Borders and the International Union Against Tuberculosis and Lung Disease, “The Life Prize: Antimicrobial Resistance Submission to the Standing Committee on Health (HESA),” October 2017, p. 2. [41] World Organization for Animal Health, Fact Sheet, “Antimicrobial Resistance.” [42] HESA, Evidence, 9 November 2017, 1535 (Dr. Duane Landals, Chari, Prudent Use Guidelines Expert Advisory Committee, Canadian Veterinary Medical Association). [43] HESA, Evidence, 13 June 2017, 1235 (Dr. Mary-Jane Ireland, Director General, Veterinary Drugs Directorate, Health Products and Food Branch, Department of Health). [47] Procalcitonin is a blood marker for bacterial infections. For more information on procalcitonin, see Schuetz P. et al, “Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections,” Cochrane Database of Systematic Reviews, 12 October 2017 [referenced in Dr. Rau’s speaking notes submitted to committee]. [49] Ibid., 1635. [50] Ibid., 1705. [52] HESA, Evidence, 21 November 2017, 1535 (Ms. Jane A. Kramer, Director, Alliance for the Prudent Use of Antibiotics). [53] HESA, Evidence, 21 November 2017, 1605 (Dr. Gerard D. Wright, Professor, Department of Biochemistry and Biomedical Sciences, McMaster University, As an Individual). [55] Government of Canada, Antimicrobial Resistance and Use in Canada: A Federal Framework for Action, October 2014. [56] Government of Canada, Tackling Antimicrobial Resistance and Antimicrobial Use: A Pan-Canadian Framework for Action, September 2017. [58] HESA, Evidence, 13 June 2017, 1135 (Ms. Aline Dimitri, Executive Director, Food Safety Science and Deputy Chief Food Safety Officer, Canadian Food Inspection Agency). [59] Government of Canada, Antimicrobial Resistance and Use in Canada: A Federal Framework for Action, October 2014. [62] Ibid. [64] Ibid. [66] Government of Canada, Tackling Antimicrobial Resistance and Antimicrobial Use: A Pan-Canadian Framework for Action. [68] Ibid. [70] Ibid., 1205. [71] HESA, Evidence, 2 November 2017, 1605 (Ms. Suzanne Rhodenizer Rose, Past President, Infection Prevention and Control Canada). [72] Ibid., 1610. [73] Ibid. [76] HESA, Evidence, 15 June 2017, 1110 (Ms. Karey Shuhendler, Policy Advisor, Policy, Advocacy and Strategy, Canadian Nurses Association). [77] HESA, Evidence, 15 June 2017, 1120 (Ms. Shelita Dattani, Director, Practice Development and Knowledge Translation, Canadian Pharmacists Association). [79] Ibid., 1145. [81] Ibid. [82] HESA, Evidence, 9 November 2017, 1545 (Mr. Steve Leech, National Program Manager, Food Safety and Animal Welfare, Chicken Farmers of Canada). [85] Ibid., 1650 (Dr. Morris. [87] Ibid. [89] See for example, HESA, Evidence, 2 November 2017, 1600 (Dr. Yoav Keynan, Scientific Lead, National Collaborating Centre for Infectious Diseases); HESA, Evidence, 7 November 2017, 1630 (Dr. Yvonne Shevchuk, Associate Dean Academic and Professor, College of Pharmacy and Nutrition, University of Saskatchewan, As an Individual); HESA, Evidence, 9 November 2017, 1410 (Dr. McEwen); 1625 (Mr. Leech). |